Hong Kong Med J 2025;31:Epub 13 Oct 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Gastroblastoma with MALAT1-GLI1 fusion gene: a case report
Yutong Sun, MD1; Huaiyin Shi, PhD2; Jiafeng Wang, MD1; Yaoqian Yuan, MD1 Xin Wu, PhD3; Yu Wang, PhD3; Jichao Pang, PhD4; Lihao Wei, PhD2; Shoulong Song, PhD5; Enqiang Linghu, PhD6; Qianqian Chen, PhD6
1 Department of Gastroenterology, Chinese People’s Liberation Army Medical School, Beijing, China
2 Department of Pathology, The First Medical Center of the People’s Liberation Army General Hospital, Beijing, China
3 Department of General Surgery, The First Medical Center of the People’s Liberation Army General Hospital, Beijing, China
4 Department of Medical Imaging, The First Medical Center of the People’s Liberation Army General Hospital, Beijing, China
5 Department of Orthopedics, Chinese People’s Liberation Army Medical School, Beijing, China
6 Department of Gastroenterology, The First Medical Center of the People’s Liberation Army General Hospital, Beijing, China
Corresponding author: Dr Qianqian Chen (Qian_Qian_Chen@163.com)
Case presentation
A 49-year-old woman presented to our hospital
with a gastric submucosal mass in March 2024.
She was asymptomatic with no abdominal signs,
and laboratory tests revealed no significant
abnormalities. Abdominal contrast-enhanced
computed tomography (CE-CT) identified a
3.2×2.2 cm2 rounded mass on the greater curvature
of the gastric antrum. The mass protruded into
and out of the cavity, displaying a clear boundary,
slightly uneven density, evident inhomogeneous
enhancement, and marked vascularity, indicative
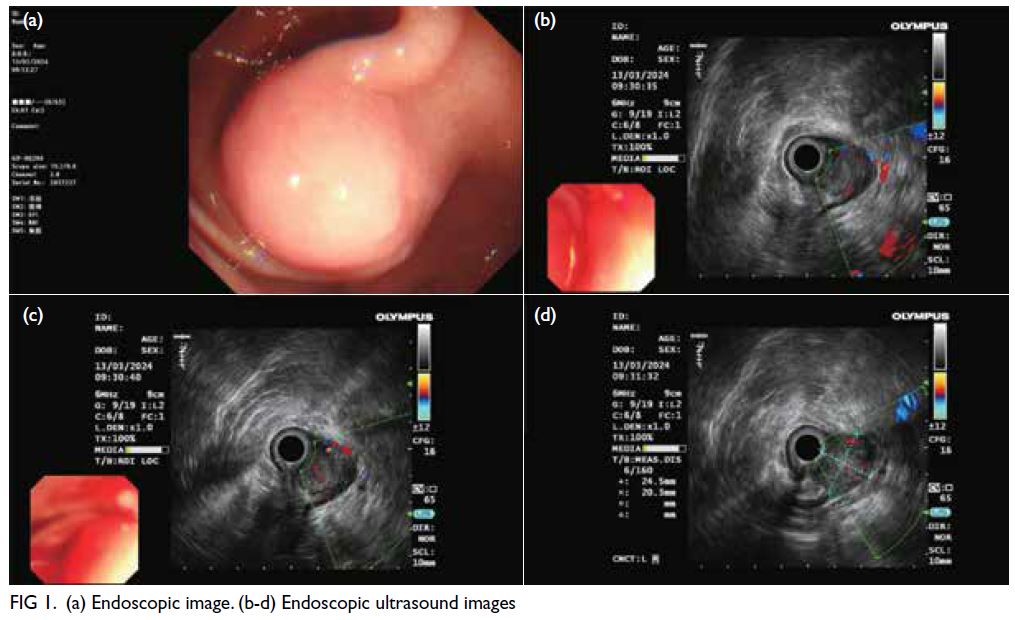
of abundant blood supply. Endoscopic ultrasound
(EUS) examination revealed a smooth-surfaced spherical bulge originating from the muscularis
propria layer. It appeared hypoechoic with areas of
hyperechoic signals, protruding into and out of the
lumen, measuring 24.5 × 20.3 mm2 in cross-section (Fig 1).
The tentative diagnosis was gastric stromal
tumour. The patient underwent laparoscopy
endoscopy cooperative surgery (LECS). During
the operation, the tumour was observed to have
an irregular shape, with no invasion or adhesion to
surrounding organs and no enlarged lymph nodes
in the abdominal cavity. The surgical specimen
revealed a tan-white solid tumour measuring
3.2×2.5×2 cm3, with a complete capsule. The margins of the specimen were clean. Postoperatively, routine
symptomatic supportive treatments such as fasting,
gastrointestinal decompression, anti-infection
therapy, acid suppression, and nutritional support
were provided. The patient recovered uneventfully.
Histological examination revealed the tumour
to be multifocally centred in the muscularis propria,
arranged in nests and cribriform patterns, with
intraluminal dense eosinophilic material. The cells
were oval, with a few fusiform in shape (Fig 2).
Immunohistochemistry revealed tumour cells to be
positive for CD56, cytokeratin, CD34, CD10, and
vimentin. The Ki-67 proliferation index was 3%. We
performed whole-transcriptome messenger RNA
sequencing of tumour tissue, which revealed a fusion
between MALAT1 (exon 1) and GLI1 (exon 3). Based
on the above evidence, the patient was definitively
diagnosed with gastroblastoma.
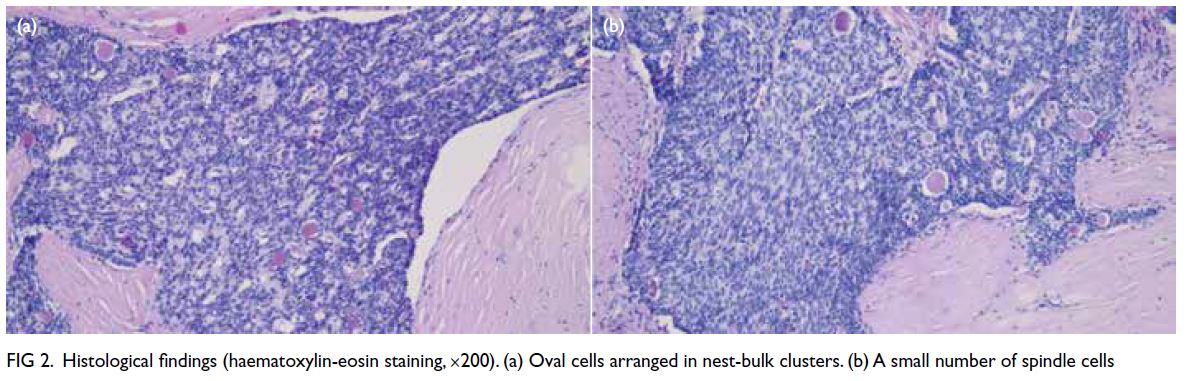
Figure 2. Histological findings (haematoxylin-eosin staining, ×200). (a) Oval cells arranged in nest-bulk clusters. (b) A small number of spindle cells
Discussion
Gastroblastoma is a rare biphasic gastric tumour
characterised by its distinctive biphasic epithelial-mesenchymal
morphology. Diagnosis from biopsy
specimens is typically challenging and often
requires additional immunohistochemistry.1 In 2009, Miettinen et al1 reported the first case of
gastroblastoma. At the time of writing, only 27 cases
had been reported.2
According to the review by Luo et al,2 the 27
patients ranged from 5 to 74 years, with a mean
age of 35 years. There were 14 male and 13 female
patients. Tumour size ranged from 1.3 to 15 cm, with
an average of 5.7 cm. Most lesions occurred in the
gastric antrum. Clinical manifestations were non-specific,
and the tumours primarily involved the
muscularis propria. Computed tomography (CT)
and EUS were the most commonly used diagnostic
methods, typically revealing a mixed solid-cystic
mass with heterogeneous hypoechoic areas, occasionally accompanied by ulcers. Two patients
underwent preoperative EUS-guided fine-needle
aspiration. In our case, the preliminary diagnosis
was gastrointestinal stromal tumour based on EUS
and CE-CT findings. Endoscopic ultrasound–guided fine-needle aspiration was not performed
to avoid damaging the tumour and increasing the
risk of metastasis. No clear associated with systemic
conditions was observed.
Gastroblastoma rarely expresses markers
typically positive in gastrointestinal stromal tumours,
solitary fibrous tumours, gastric schwannomas, or
mesotheliomas. It also generally lacks expression
of markers characteristic of gastric neuroendocrine
tumours or leiomyomas.3 In the 27 reported cases,2
most tumour cells were positive for vimentin, CD56,
CD10, and PCK. The MALAT1-GLI1 fusion gene
was detected in nine patients and is considered a
valuable diagnostic marker for this tumour.2
Only three cases presented with organ or lymph
node metastasis.2 One patient had peritoneal, liver,
and pelvic metastases, as well as bladder adhesion
and lymph node metastasis at diagnosis. Following
partial gastrectomy, no recurrence or metastasis was
observed at 3-month follow-up. Another patient
had two lymph node metastases at the splenic hilum
prior to surgery. After partial gastrectomy and
splenectomy, local recurrence was noted at 6 months,
and surgical debulking was performed. Another
patient had liver metastasis preoperatively, but no
postoperative follow-up information was available.
Among the other surgically treated patients, only one
experienced local recurrence. These findings suggest
that while gastroblastoma may exhibit indolent
behaviour, preoperative metastasis or invasion may
be associated with a poor prognosis.
Surgery remains the mainstay of treatment.
Of the 27 cases,2 23 underwent surgical resection
and three received endoscopic treatment. The mean
tumour size in the surgical group was 5.80 cm, compared to 1.91 cm in the endoscopic treatment
group, suggesting that tumour size influenced
the choice of treatment modality.2 Among the
endoscopically treated patients, one underwent
endoscopic full-thickness resection, and two
underwent endoscopic submucosal dissection.2 The
choice of endoscopic resection generally depends on
lesion depth, size, and location. When endoscopic
resection is not feasible, laparoscopy endoscopy
cooperative surgery may be considered.4 In our case,
laparoscopy endoscopy cooperative surgery was used,
offering the benefits of minimally invasive surgery
while reducing postoperative complications. In one
previous case, a patient with a 15-cm gastroblastoma
underwent postoperative radiotherapy and remained
disease-free after 14 years of follow-up.2 The
remaining patients received routine postoperative
follow-up.2 Given the potential for recurrence,
particularly in cases with preoperative metastasis
or invasion, adjuvant radiotherapy or chemotherapy
may be considered on an individual basis. The
average follow-up duration across reported cases
was 31 months.2 Given its generally indolent nature,
annual follow-up is recommended for most patients
with gastroblastoma. For those with preoperative
metastasis or invasion, more frequent follow-up
every 3 to 6 months is advisable. As gastroblastoma
remains rare, additional case reports and studies are
needed to enhance our understanding of its biological
behaviour, diagnosis, and optimal management.
Author contributions
Concept or design: Q Chen.
Acquisition of data: Y Sun, H Shi, X Wu, Y Wang, E Linghu.
Analysis or interpretation of data: Y Sun, H Shi, J Wang, Y Yuan, J Pang, L Wei, S Song.
Drafting of the manuscript: Y Sun.
Critical revision of the manuscript for important intellectual content: Q Chen.
Acquisition of data: Y Sun, H Shi, X Wu, Y Wang, E Linghu.
Analysis or interpretation of data: Y Sun, H Shi, J Wang, Y Yuan, J Pang, L Wei, S Song.
Drafting of the manuscript: Y Sun.
Critical revision of the manuscript for important intellectual content: Q Chen.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study was funded by the 14th Five-year National Key Research and Development Program of China (Project No.: 2022YFC2503600). The funder had no role in study design,
data collection, analysis, interpretation, or manuscript
preparation.
Ethics approval
This study was approved by the Ethics Committee of Chinese
People’s Liberation Army General Hospital, China (Ref No.: S2023-188-01). The patient was treated in accordance with
the Declaration of Helsinki and provided written informed
consent for all treatments, procedures and the publication of
this case report.
References
1. Miettinen M, Dow N, Lasota J, Sobin LH. A distinctive
novel epitheliomesenchymal biphasic tumor of the
stomach in young adults (“gastroblastoma”): a series of 3
cases. Am J Surg Pathol 2009;33:1370-7. Crossref
2. Luo Z, Cui J, Ma F, et al. Gastroblastoma—a case report and literature review. World J Surg Oncol 2024;22:255. Crossref
3. Chen C, Lu J, Wu H. Case report: submucosal gastroblastoma with a novel PTCH1::GLI2 gene fusion in a 58-year-old man. Front Oncol 2022:12:935914. Crossref
4. Sharzehi K, Sethi A, Savides T. AGA clinical practice
update on management of subepithelial lesions
encountered during routine endoscopy: expert review.
Clin Gastroenterol Hepatol 2022;20:2435-43.e4. Crossref