Hong Kong Med J 2025;31:Epub 11 Jul 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Mixed laterally spreading tumour and
neuroendocrine tumour in the rectum: a case report
Weijie Zhou#, MM, Xueping Ke#, MD, Yuxuan Lin, MM, Guoyin Li, MM, Mingyun Zheng, MM, Guoxin Liufu, MM, Liu Liu, MM
Department of Gastroenterology, The Six Affiliated Hospital of South China University of Technology, Guangdong, China
# Equal contribution
Corresponding author: Mr Liu Liu (liuliu8495@163.com)
Case presentation
A 58-year-old female presented to our hospital
with 1-year history of recurrent mucous stools.
She had no significant medical or family history of
cancer. Laboratory tests for intestinal pathogens,
rheumatological markers, and tumour markers were
all within normal limits. Abdominal imaging did not
reveal any abnormalities. Colonoscopy showed a
laterally spreading tumour measuring approximately
25 mm × 40 mm located about 5 cm from the anal
verge. The tumour exhibited granular, nodular, and
lobulated features with abundant mucus adhering
to the surface (Fig 1a). Despite repeated washing,
mucus remained attached to the tumour surface.
Subsequently, we performed indigo carmine
staining, which revealed well-delineated tumour
margins (Fig 1b). Endoscopic ultrasound showed the lesion originated from the mucosal layer. A local
biopsy revealed a tubulovillous adenoma with high-grade
dysplasia. The patient was deemed suitable for
endoscopic submucosal dissection (Fig 1c to e).
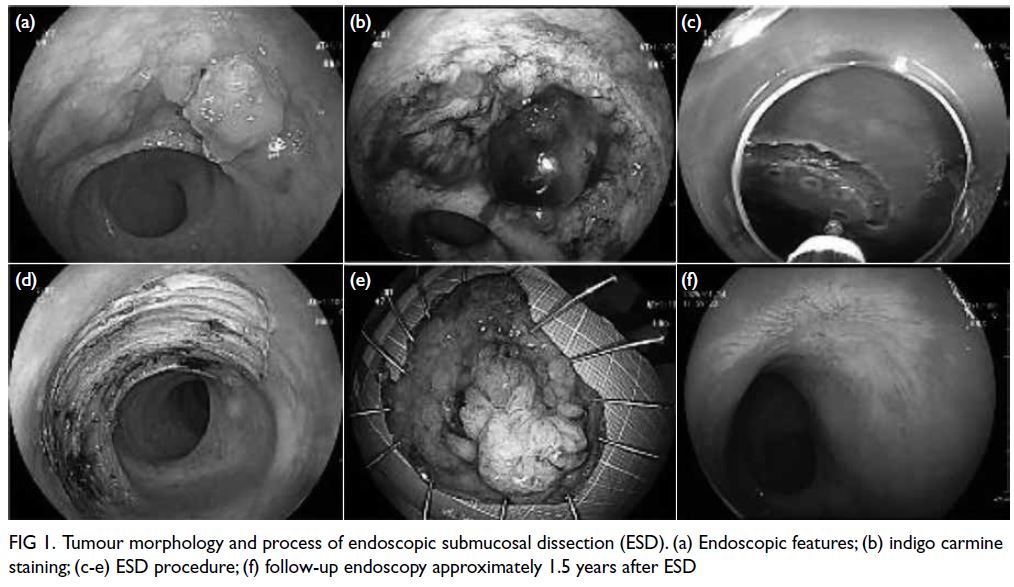
Figure 1. Tumour morphology and process of endoscopic submucosal dissection (ESD). (a) Endoscopic features; (b) indigo carmine staining; (c-e) ESD procedure; (f) follow-up endoscopy approximately 1.5 years after ESD
Whole tumour pathology was highly unusual,
which showed a combination of tubulovillous adenoma with high-grade
dysplasia and a neuroendocrine neoplasm
(NEN) component. Interestingly, the neuroendocrine
tumour had a maximum diameter of approximately
0.3 cm, representing around 3% of the lesion.
Immunohistochemistry staining revealed positive
expression of CK, Syn, CD56, CgA, Ki-67 (<1%) and
CD34 (Fig 2). This pathological manifestation did
not align with the current classification of NENs.
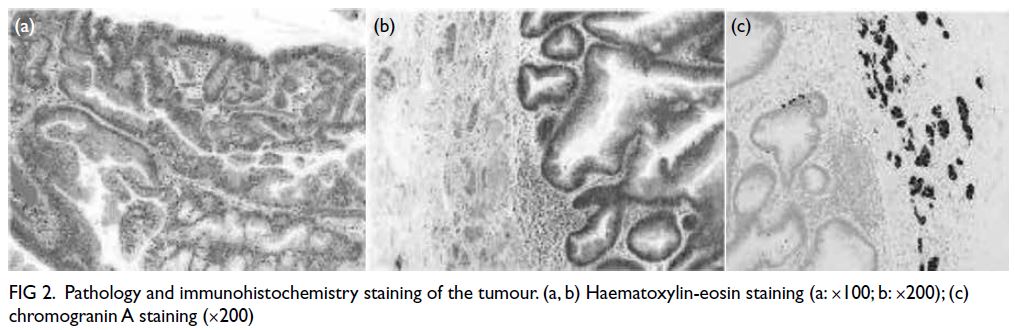
Figure 2. Pathology and immunohistochemistry staining of the tumour. (a, b) Haematoxylin-eosin staining (a: ×100; b: ×200); (c) chromogranin A staining (×200)
About 1.5 years postoperatively,
colonoscopy showed a scar at the site of the
previous rectal procedure (Fig 1f). Enhanced chest and abdominal computed tomography scans
showed slight thickening of the rectal mucosa
without evidence of regional or distant lymph node
enlargement.
Discussion
Neuroendocrine neoplasms are a rare type of tumour
and encompass three major subtypes: neuroendocrine
tumours, neuroendocrine carcinomas and mixed
neuroendocrine–non-neuroendocrine neoplasms
(MiNEN). Among these, MiNENs are a special
type with high invasiveness. Our case resembled a
MiNEN but exhibited some distinct differences.
In this case, the pathology was special. It did not
align with the current World Health Organization
classification of NENs.1 These neoplasms, known
as MiNENs, are characterised by a combination
of neuroendocrine and non-neuroendocrine
components, both of which comprise at least 30% of
the neoplasm.1 Although our case shared similarities
with MiNENs in terms of mixed histology, it differed
significantly in the proportion of components, with
the neuroendocrine tumour component constituting
less than 30%. Evidently, this case did not meet the
current definition of MiNENs. In fact, the definition
of MiNENs remains controversial.
These mixed tumours (neuroendocrine–non-neuroendocrine
neoplasms) were first described
in 1924.2 In 2000, a classification system for
endocrine tumours was implemented and defined
mixed exocrine–endocrine carcinomas as tumours
in which each component constitutes at least
30% of the neoplasm.2 In 2010, the World Health
Organization classified mixed neuroendocrine and
exocrine tumours as mixed adenoneuroendocrine
carcinomas.2 Subsequently, in 2017, mixed
adenoneuroendocrine carcinomas were reclassified
as MiNENs. The term “exocrine” was replaced with
“non-neuroendocrine” to encompass a broader
range of possible histological variants, including
glandular, squamous, mucinous, and sarcomatoid
phenotypes.3 As for the threshold of at least 30% for each component, it is highly unusual for a
component with a lower representation to affect
the biological behaviour of a cancer.2 Nonetheless,
the threshold was arbitrarily set without clinical
or scientific evidence.4 Given the emergence of our
case, we believe that this threshold requires further
optimisation.
Regarding the pathology in our patient, we
proposed the following explanations. First, there
are two widely accepted hypotheses for the origin
of MiNENs.5 6 7 8 The first posits that both tumour
components originate from a single precursor
cell but proliferate and differentiate along distinct
pathways. The second hypothesis also suggests a
common cellular origin. Nonetheless, it proposes
that during tumour progression, a subset of the non-neuroendocrine
component accumulates sufficient
genetic mutations to transform into neuroendocrine
cells. These theories suggest that the composition
of MiNENs is dynamic, with potentially varying
proportions of components at different stages
of tumour development. Second, with growing
health awareness and the widespread adoption of
endoscopic screening, early-stage tumours are more
readily identified. These early-stage neoplasms are
typically smaller in size and exhibit a lower degree
of malignancy. These factors collectively contribute
to the evolving landscape of MiNENs diagnosis and
classification, necessitating ongoing refinement of
diagnostic criteria and classification systems.
In terms of endoscopic manifestation, there
was something worth considering. In this case,
the surface of the tumour was repeatedly washed,
but mucus adhesion persisted, more similar to the
manifestation of mucinous adenocarcinoma or
serrated adenocarcinoma.9 Notably, the absence
of classic carcinoid syndrome symptoms and
negative tumour markers further set this case
apart. Although villous tubular adenomas can
secrete mucus, the tumour in this case exhibited
unusually copious and rapid mucus production.
We suspected the neuroendocrine tumour may
possess paracrine functions that further stimulated secretion from the adenoma. Nonetheless, there
have been no experiments supporting this viewpoint.
Experimental validation in the future is needed
to elucidate the potential interplay between these
neoplastic entities and their secretory mechanisms.
In terms of treatment, although a definitive
classification of this tumour type has not been
established, the existing treatment principles for
NENs remain applicable. For this patient, the
neuroendocrine tumour lesion was less than 10 mm
in size, with a Ki-67 index of less than 3%, classifying
it as a G1 stage tumour, and there was no evidence
of metastasis to other organs or tissues. We
performed endoscopic submucosal dissection to
remove the tumour. Nonetheless, it was important
to consider the depth of resection. Resection above
the muscularis mucosae may result in incomplete
tumour removal, while excision below this layer risks
vascular injury. We recommended resection close to
the muscularis mucosa to minimise bleeding and to
prevent tumour seeding into blood vessels. Another
critical consideration was the extent of resection. It
was imperative to ensure negative tumour margins
to guarantee complete excision of the neoplasm.
Our case indicates that the current classification
system for NENs remains inadequate. Specifically,
there is no clear classification for tumours that
contain a minor component of neuroendocrine cells,
highlighting an urgent need for further refinement
of MiNENs.
Author contributions
Concept or design: W Zhou, X Ke.
Acquisition of data: W Zhou, X Ke.
Analysis or interpretation of data: W Zhou, X Ke.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: L Liu.
Acquisition of data: W Zhou, X Ke.
Analysis or interpretation of data: W Zhou, X Ke.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: L Liu.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki. The patient provided informed consent for all procedures agreement for publication of this article.
References
1. Washington MK, Goldberg RM, Chang GJ, et al. Diagnosis
of digestive system tumours. Int J Cancer 2021;148:1040-50. Crossref
2. Díaz-López S, Jiménez-Castro J, Robles-Barraza CE, Ayala-de
Miguel C, Chaves-Conde M. Mixed neuroendocrine
non-neuroendocrine neoplasms in gastroenteropancreatic
tract. World J Gastrointest Oncol 2024;16:1166-79. Crossref
3. Kanthan R, Tharmaradinam S, Asif T, Ahmed S, Kanthan SC.
Mixed epithelial endocrine neoplasms of the colon and
rectum—an evolution over time: a systematic review.
World J Gastroenterol 2020;26:5181-206. Crossref
4. Toor D, Loree JM, Gao ZH, Wang G, Zhou C. Mixed
neuroendocrine–non-neuroendocrine neoplasms of the
digestive system: a mini-review. World J Gastroenterol
2022;28:2076-87. Crossref
5. Frizziero M, Chakrabarty B, Nagy B, et al. Mixed
neuroendocrine non-neuroendocrine neoplasms: a
systematic review of a controversial and underestimated
diagnosis. J Clin Med 2020;9:273. Crossref
6. Bazerbachi F, Kermanshahi TR, Monteiro C. Early
precursor of mixed endocrine-exocrine tumors of
the gastrointestinal tract: histologic and molecular
correlations. Ochsner J 2015;15:97-101.
7. Scardoni M, Vittoria E, Volante M, et al. Mixed
adenoneuroendocrine carcinomas of the gastrointestinal
tract: targeted next-generation sequencing suggests
a monoclonal origin of the two components.
Neuroendocrinology 2014;100:310-6. Crossref
8. Yuan W, Liu Z, Lei W, et al. Mutation landscape and intra-tumor
heterogeneity of two MANECs of the esophagus
revealed by multi-region sequencing. Oncotarget
2017;8:69610-21. Crossref
9. Lee CT, Huang YC, Hung LY, et al. Serrated adenocarcinoma
morphology in colorectal mucinous adenocarcinoma is
associated with improved patient survival. Oncotarget
2017;8:35165-75. Crossref

