© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Clinical and molecular features of pleuropulmonary blastoma in children in Hong Kong: case reports
Anthony PY Liu, MB, BS1; Marcus KL Fung, PhD1; Mianne Lee, MSc1; Jasmine LF Fung, BSc1; Mandy HY Tsang, MMedSc1; CW Luk, MB, BS2; Brian HY Chung, MB, BS, MD1; Godfrey CF Chan, MD1
1 Department of Paediatrics and Adolescent Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong
2 Department of Paediatrics and Adolescent Medicine, Hong Kong Children’s Hospital, Hong Kong
Corresponding author: Prof Godfrey CF Chan (gcfchan@hku.hk)
Case report
Patient 1
In January 1998, a 14-month-old girl presented
with fever and dyspnoea. Plain chest radiograph
showed opacification of the right hemithorax that
corresponded to a multicystic mass on computed
tomography. Thoracotomy was performed and a
11-cm × 9.5-cm × 5-cm mixed cystic-solid mass
excised. Together with histological findings, type II
pleuropulmonary blastoma (PPB) was diagnosed.
The patient was treated with adjuvant chemotherapy
according to the Intergroup Rhabdomyosarcoma
Study-IV regimen but developed ifosfamide-induced
renal tubulopathy. At the time of writing,
the patient remains in remission (age 23 years).
Sanger sequencing revealed no DICER1 mutation in
the patient’s peripheral blood DNA.
Patient 2
In November 2008, a 9-month-old girl presented
with progressive dyspnoea. Plain chest radiograph
revealed left-sided pneumothorax. Computed tomography scan of the thorax after thoracocentesis
revealed a 3-cm cystic lesion in the lingula, suspicious
of congenital cystic adenomatoid malformation.
In view of the risk of recurrent pneumothorax, left
upper lobectomy was performed. Microscopically,
features of the excised lesion were compatible
with type I PPB. No adjuvant treatment was
required and the patient remained in remission
for 10 years. Multinodular goitre (MNG) was
also diagnosed at age 7 years. Sanger sequencing
of the patient’s peripheral blood DNA revealed
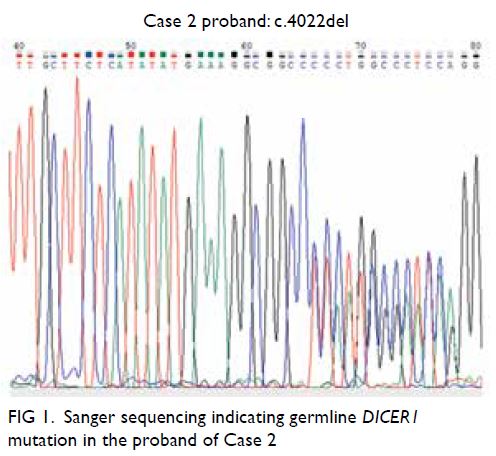
a heterozygous frameshift mutation in DICER1
(NM_177438.2:c.4022del(p.(Gly1341Alafs*6)); Fig 1),
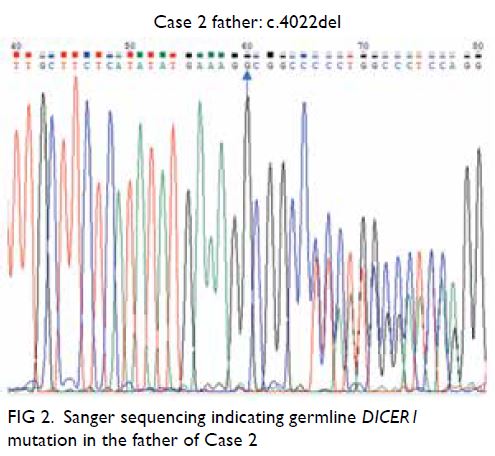
inherited from her father (Fig 2), who also had a
history of MNG with thyroidectomy done. Familial
counselling was offered with discussion about the
role of further cascade testing and recommendations
for surveillance.
Patient 3
In December 1999, a 2-year-old girl presented with presumed right-sided pneumonia unresolved for
2 months. Computed tomography scan of the thorax showed collapse-consolidation with pleural effusion.
Exploratory thoracotomy revealed a huge mass in
the right middle lobe and debulking was performed.
Histology confirmed type II PPB. A new computed
tomography scan of the thorax demonstrated a
residual multiloculated cystic mass with intermixed
solid components. Adjuvant chemotherapy based
on the Intergroup Rhabdomyosarcoma Study-IV
regimen was adopted. Right middle lobectomy
was performed after week 8 of chemotherapy. The
patient subsequently developed cerebral relapses at
age 7 years and again at age 17 years for which she
was treated with excision and chemoradiotherapy
(ifosfamide/carboplatin/etoposide + 40-Gy focal
radiotherapy and irinotecan/temozolomide +
40-Gy focal radiotherapy, respectively). At the time
of writing, the patient (age 21 years) has been in
remission for 4 years. Multinodular goitre was also
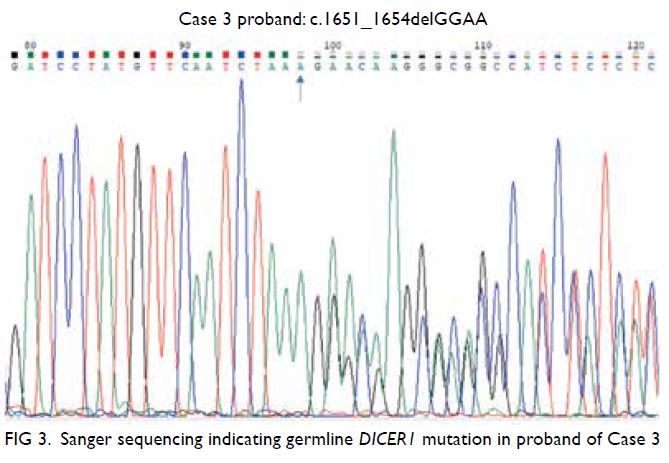
diagnosed at age 13 years. A heterozygous frameshift
mutation in DICER1 was detected in the patient’s
peripheral blood using Sanger sequencing (NM_17
7438.2:c.1651_1654delGGAA(p(Gly551Glufs*10));
Fig 3).
Patient 4
In January 2016, a 2-year-old girl presented with fever, cough and progressive dyspnoea. Computed
tomography of the thorax revealed a large space-occupying
lesion in the right thoracic cavity, with
pleural effusion, mediastinal shift and superior
vena cava compression. Biopsy confirmed type
III PPB. Considering the risk of upfront surgery,
neoadjuvant chemotherapy with ifosfamide,
vincristine, actinomycin and doxorubicin was
started. Unfortunately her response was suboptimal
and she developed respiratory embarrassment
and required emergency surgery during which
she went into cardiopulmonary arrest. Despite
successful resuscitation and debulking surgery, the patient demonstrated signs of severe hypoxic
ischaemic encephalopathy. In view of the poor
neurodevelopmental prognosis, the family elected to
continue only palliative care. The patient eventually
succumbed to progression 6 months after diagnosis.
Sanger sequencing of peripheral blood DNA
showed a nonsense mutation on exon 7 of DICER1
(NM_177438.2:c.1174C>T, p.R392*; Fig 4); her father
tested negative for the same germline mutation and
her mother was not available for evaluation.
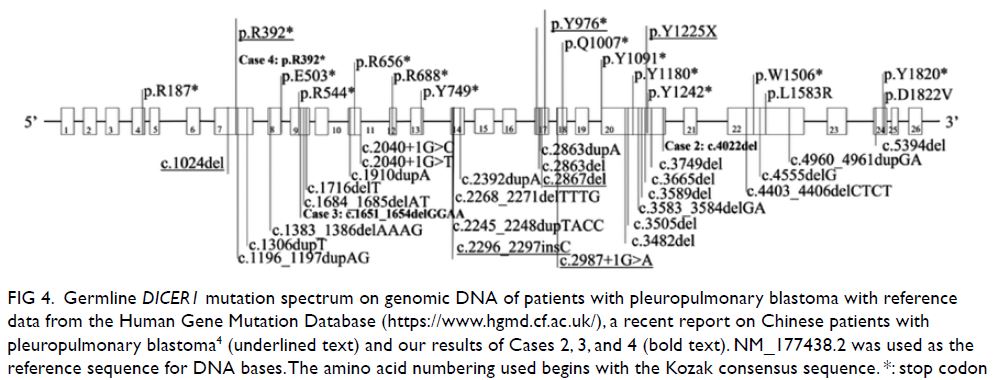
Figure 4. Germline DICER1 mutation spectrum on genomic DNA of patients with pleuropulmonary blastoma with reference data from the Human Gene Mutation Database (https://www.hgmd.cf.ac.uk/), a recent report on Chinese patients with pleuropulmonary blastoma4 (underlined text) and our results of Cases 2, 3, and 4 (bold text). NM_177438.2 was used as the reference sequence for DNA bases. The amino acid numbering used begins with the Kozak consensus sequence. *: stop codon
Discussion
Pleuropulmonary blastoma is a rare thoracic tumour that arises during infancy or young childhood.1
Its aggressiveness and presenting features vary
according to the histological subtype, ranging from
the self-limiting cystic form (type I) with potential
to undergo spontaneous regression (type Ir) or the mixed cystic/solid form (type II) that would
benefit from adjuvant cytotoxic treatment, to a
solid form (type III) that carries a considerable
risk of disease relapse despite multimodal therapy.
As an entity originally designated in 1988, familial
clustering of PPB was first reported in 1996. This
led to the recognition of underlying germline
DICER1 alterations, and subsequently the definition
of DICER1 syndrome—an autosomal dominant
condition with variable penetrance and associated
with almost 30 neoplastic conditions, including
MNG, cystic nephroma, and ovarian stromal
tumours.2 The oncogenic mechanism consequent to
DICER1 mutations is hypothesised to be related to
the resulting imbalance of mature miRNAs derived
from the 5′ and 3′ ends of the precursor pre-miRNA.
Because of its rarity, data on the presentation and
molecular features of patients with PPB in Chinese
are limited and that in Hong Kong have never been
reported.
We reviewed a territory-wide paediatric
oncology database and report the various presenting
features and clinical course of four patients with
PPB treated between 1998 and 2020. Type I/type
Ir PPB should be considered in cases of cystic lung
lesions; while many represent as incidental findings,
the risk of pneumothorax remains a concern for
peripherally located lesions. Among those where
resection has been performed, 90% will remain
progression-free without the use of adjuvant
chemotherapy.1 Type II and III PPB are differential
diagnoses for young children (age <6 years) who
present with a space-occupying thoracic mass or
apparent, persistent chest infection. The surgical
and perioperative management of massive lung
lesions with mediastinal compression carries a high-risk
of cardiovascular compromise and necessitates
care at a tertiary referral centre with available
expertise including extracorporeal membrane
oxygenation support. For adjuvant therapy, the
addition of doxorubicin to ifosfamide, vincristine
and actinomycin has been shown to be efficacious
in Types II/III PPB.3 However, half of these patients
still develop progression by age 5 years, 60% with a
central nervous system component.
The prevalence of germline pathogenic DICER1 mutations in the general population is estimated
to be 1:10 600 in the ExAC-nonTCGA (The Cancer
Genome Atlas) database, although mutations have
been identified in two-thirds of patients with PPB with
thus far a lack of genotype-phenotype correlation.
In our series, novel heterozygous germline DICER1
frameshift mutations were found in cases 2 and 3;
while a heterozygous germline nonsense mutation,
reported recently in another Chinese patient, was
detected in Case 4 (Fig 4).4 The findings of our
case series add to the spectrum of known DICER1
mutations, especially to the very limited data from Asia.4 Diagnosing DICER1 syndrome facilitates
surveillance of associated morbidities, familial
testing and reproductive counselling for both
probands and symptomatic carriers.5 Further studies
and consideration of a prospective patient registry
to define the prevalence of DICER1-associated
conditions in both paediatric and adult populations
in Hong Kong are warranted.
Author contributions
Concept or design: APY Liu, MKL Fung, BHY Chung, GCF Chan.
Acquisition of data: All authors.
Analysis or interpretation of data: APY Liu, MKL Fung, M Lee, JLF Fung.
Drafting of the manuscript: APY Liu, MKL Fung.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: APY Liu, MKL Fung, M Lee, JLF Fung.
Drafting of the manuscript: APY Liu, MKL Fung.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
We would like to thank the patients and family for their consent to this study.
Funding/support
This study was supported by The Society for the Relief of Disabled Children. The funder had no role in study design, data collection/analysis/interpretation or manuscript preparation.
Ethics approval
This study was approved by the Institutional Review Board of the University of Hong Kong (Ref No. UW12-211) with
informed consent obtained from patient guardians.
References
1. Messinger YH, Stewart DR, Priest JR, et al. Pleuropulmonary
blastoma: a report on 350 central pathology-confirmed
pleuropulmonary blastoma cases by the International
Pleuropulmonary Blastoma Registry. Cancer 2015;121:276-85. Crossref
2. de Kock L, Wu MK, Foulkes WD. Ten years of DICER1 mutations: provenance, distribution, and associated
phenotypes. Hum Mutat 2019;40:1939-53. Crossref
3. Doros LA, Schultz KA, Harris A, et al. IVADo treatment
of type II and type III pleuropulmonary blastoma (PPB):
a report from the International PPB Registry. J Clin Oncol
2014;32(15 Suppl):10060. Crossref
4. Cai S, Wang X, Zhao W, Fu L, Ma X, Peng X. DICER1 mutations in twelve Chinese patients with pleuropulmonary blastoma. Sci China Life Sci 2017;60:714-20. Crossref
5. Schultz KA, Williams GM, Kamihara J, et al. DICER1 and associated conditions: identification of at-risk individuals and recommended surveillance strategies. Clin Cancer Res 2018;24:2251-61. Crossref