Hong Kong Med J 2025;31:Epub 25 Jul 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
EDITORIAL
Importance of surveillance and vaccination in
managing respiratory syncytial virus infections among older adults in Hong Kong
Jane CK Chan, MD (UChicago), DABIM1; Mike YW Kwan, MSc, MRCPCH1,2; Wilson Lam, FRCP (Edin), FHKAM (Medicine)3; Christopher KC Lai, FRCPath (UK), FHKAM (Pathology)4,5; Grant Waterer, FRACP, FCCP6; Anna Cheng, FHKAM (Paediatrics), MPH (CUHK)7; Maureen Wong, FHKAM (Medicine)8; KM Sin, FHKAM (Medicine)9; Ken KP Chan, FHKAM (Medicine), FRCP (Glasg)10,11; Angus Lo, FHKAM (Medicine), FRCP (Edin)12; Macy MS Lui, MD, FHKAM (Medicine)13; WS Leung, FHKAM (Medicine)14; Martin CS Wong, MD, MPH15,16
1 Hong Kong Chinese Medical Association Ltd, Hong Kong SAR, China
2 Hong Kong Hospital Authority Infectious Disease Centre, Princess Margaret Hospital, Hong Kong SAR, China
3 Specialist in Infectious Disease, Private Practice, Hong Kong SAR, China
4 Department of Microbiology, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
5 SH Ho Research Centre for Infectious Diseases, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
6 Royal Perth Hospital, University of Western Australia, Perth, Australia
7 Department of Paediatrics and Adolescent Medicine, United Christian Hospital, Hong Kong SAR, China
8 Department of Medicine and Geriatrics, Caritas Medical Centre, Hong Kong SAR, China
9 Department of Medicine and Geriatrics, Tuen Mun Hospital, Hong Kong SAR, China
10 Department of Medicine and Therapeutics, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
11 Li Ka Shing Institute of Health Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
12 Specialist in Respiratory Medicine, Private Practice, Hong Kong SAR, China
13 Division of Respiratory Medicine, Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Hong Kong SAR, China
14 Department of Medicine and Geriatrics, Kwong Wah Hospital, Hong Kong SAR, China
15 The Jockey Club School of Public Health and Primary Care, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
16 Editor-in-Chief, Hong Kong Medical Journal
Corresponding author: Dr Jane CK Chan (finehealth@gmail.com)
Respiratory syncytial virus: an
underrecognised and evolving public health threat
Respiratory syncytial virus (RSV) is a common
cause of respiratory infections globally and in Hong
Kong.1 2 It is the leading cause of hospitalisation due
to respiratory viral infections among infants and
young children, and it is increasingly recognised
as a substantial threat to older adults.1 2 3 4 Although
hospitalised at lower rates than infants and young
children, older people are more likely to experience
severe outcomes, including cognitive decline,
infection-triggered acute myocardial infarction,
stroke, or even death.1 2 3 4 Contemporary data
suggest that the spread and consequences of RSV,
particularly in older adults, have consistently been
underestimated.1 2 4 In older adults, the clinical
outcomes of RSV infection are comparable to, or
even more severe than, those of influenza.2 3 In Hong
Kong, large-scale epidemiological data on RSV are
currently unavailable.
Both upper respiratory tract and lower
respiratory tract (LRT) specimens may be used to test for RSV. In a previous local study reviewing multiplex
polymerase chain reaction (PCR) results from 20 127
respiratory specimens tested in a hospital across
all age-groups between 2014 and 2023, RSV was
detected in 2.03% of LRT specimens (including sputa
and endotracheal/tracheal/bronchial aspirates)
and in 7.93% of upper respiratory tract specimens
(combined nasal/nasopharyngeal and throat swabs).5
In a multicentre, prospective study recruiting
adult patients with chronic obstructive pulmonary
disease and infective exacerbations, a higher RSV
positivity rate by quantitative PCR was observed
in sputum samples compared with nasopharyngeal
swabs (7.69% vs 2.02%, respectively).6 Given that
there is no single RSV test in adults with acceptable
diagnostic accuracy, these figures may represent
underestimates.6 In this study, 59% of RSV-associated
exacerbations were PCR-negative, despite sample
collection within 5 days of symptom onset.6
Local studies have demonstrated substantial
morbidity and mortality associated with RSV
infections among older adults. A small study found
that, of 71 older adults (median age 75 years; 74%
with comorbidities) hospitalised with RSV, 61% required supplemental oxygen, and 18% had severe
disease requiring non-invasive ventilation or
intensive care, or resulting in death within 30 days.7
Furthermore, in a retrospective study of adults
admitted between 2009 and 2011 to three acute care
general hospitals in Hong Kong serving a population
of over 1.5 million, 607 patients (mean age 75 years)
had virologically confirmed RSV infection; 30-and 60-day mortality rates were 9.1% and 11.9%,
respectively.3 Finally, in a study of hospitalised
patients with laboratory-confirmed respiratory virus
infections between 1998 and 2012, the incidence
of hospitalisation due to RSV was 2.09 per 10 000
population in both men and women aged 65 to 74
years. The mean annual mortality in this age-group
was 17.44 per 1 000 000 population for men and
11.02 per 1 000 000 population for women.1
A disruption in the population genetic diversity
and seasonality of RSV as a consequence of the
COVID-19 pandemic has been observed.8 9 10
Australian data demonstrated that many historically
detected RSV lineages were no longer circulating
after 2021, having been superseded by two novel
RSV-A lineages, which may become dominant due
to their greater resilience, fitness, infectivity, or a
combination of these factors.8 Additionally, the RSV
Hospitalization Surveillance Network in the United
States has shown that COVID-19 affected RSV
seasonality, with a shorter but more intense season of
infection in 2022-2023, though with at least a trend
towards pre-COVID norms in the 2023-2024 season.9
Although surveillance for RSV in Hong Kong
is comparatively less extensive, data from the local
health authority suggest that the post-COVID
pattern of RSV has also changed; it may now comprise
a single infective season peaking between April
and October.10 Moreover, a recent local paediatric
study showed significantly increased odds of RSV
infection in children aged 3 years or above after the
COVID-19 lockdown, although the full impact
remains to be determined.11
In addition to the evolving pathogenicity of
RSV infection following the COVID-19 pandemic,
it is important to note the increasingly ageing
population and, consequently, a growing vulnerable
population in regions such as Hong Kong, which
will inevitably contribute to a heavier RSV disease
burden.12 Accordingly, it is crucial to conduct
adequate surveillance to monitor the changing
epidemiology and to inform appropriate risk
mitigation strategies.
Along with increased surveillance, the
recent introduction of vaccines against RSV (from
2023 onwards) has created a new opportunity to
manage the risk of infection and its consequences.13
Internationally, vaccination against RSV has
been recommended by national or supranational
organisations in multiple locations; older age is recognised as an independent risk factor, alongside
various cardiopulmonary, metabolic, and immune
conditions (Table).2 13 Understanding the extent of
risk for the local population in Hong Kong, as well as
the effectiveness of targeted interventions (eg, vaccine
rollout), will rely on analyses of epidemiological data.
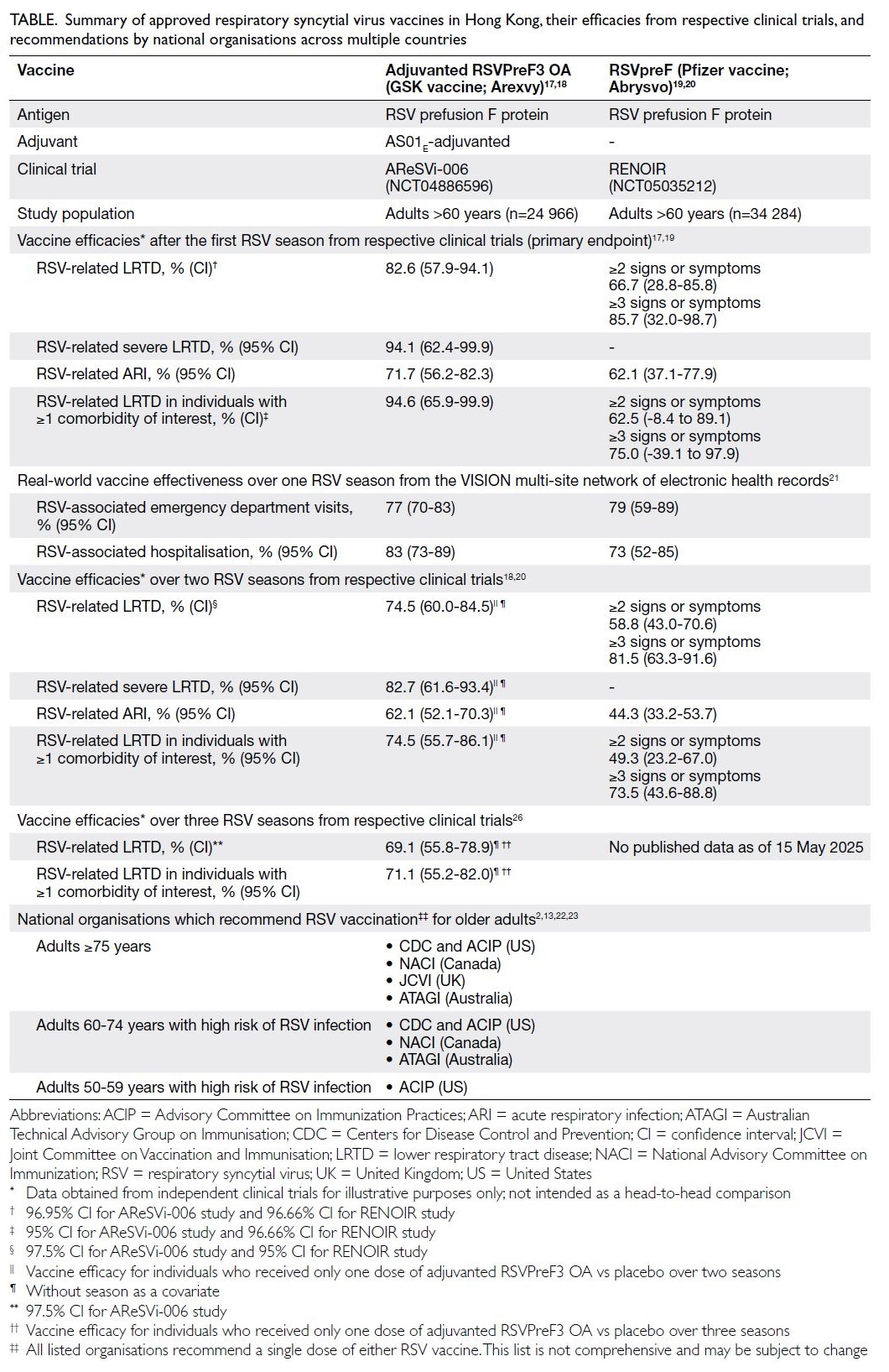
Table. Summary of approved respiratory syncytial virus vaccines in Hong Kong, their efficacies from respective clinical trials, and recommendations by national organisations across multiple countries
Respiratory syncytial virus surveillance: international approaches and implications for Hong Kong
Globally, approaches to RSV surveillance vary
considerably. In Australia and South Korea, RSV
is actively monitored as a notifiable disease, and
healthcare professionals (HCPs) are mandated to
report all confirmed cases to a central source.14 15
Active surveillance promotes testing for RSV, while
its status as a notifiable disease facilitates systematic
data collection, which, upon analysis, can effectively
support the formulation of health strategies, their
implementation, and informed health policy
decision making.
Given that RSV is not a notifiable disease,
its surveillance in Hong Kong is primarily
conducted through sentinel clinics and a limited
number of public and private hospitals. Sentinel
systems generally require relatively low resource
consumption but may not cover a sufficiently large
population to provide accurate estimates of virus
circulation. The impetus to test for RSV may also
be limited in patients presenting with non-severe
respiratory illnesses, which are common, exhibit
non-specific symptoms, and are typically managed
in a similar manner regardless of aetiology.15 Thus,
where most sentinel activity occurs in primary care
or community settings, the true burden may be
underrepresented and skewed towards less severe
cases. In contrast, sentinel systems in hospital
settings can yield more detailed information on
severe cases and outcome data concerning the
most serious consequences of infection.15 In Hong
Kong, RSV is likely underdiagnosed, partly due to
low testing rates in adults, and partly due to skewed
reporting by the existing sentinel system.
To enhance the RSV testing rate, educational
campaigns for HCPs should be implemented to
increase clinical suspicion of RSV in adults and
to raise awareness of its potential consequences
in older adults and other high-risk populations
(eg, those with chronic respiratory, cardiac,
endocrine, or renal diseases, as well as those with
immunodeficiency).12 13 Targeted surveillance in
these patient groups may offer cost savings. Testing
sites can also be prioritised in areas of greatest risk,
assessing the penetration and spread of RSV among
populations such as older adults residing in higher-density
settings, including long-term care facilities.
To mitigate the resource and workload
implications of increased testing, European and
other international guidelines have recommended
incorporating RSV into existing surveillance systems
for respiratory infections.15 16 Furthermore, it is
important to standardise the testing method5 12 15;
PCR remains the preferred tool for confirming
RSV cases, given the risk of false-negative results
with current rapid antigen tests. Hospital-based
surveillance, covering nasopharyngeal specimens
and beyond, may be considered, particularly to
address the potential underdiagnosis of severe RSV
cases.
Practical considerations for enhancing RSV
surveillance in Hong Kong may include integration
with broader respiratory pathogen surveillance and
diagnostic systems, such as those for COVID-19
and influenza.15 Moreover, it is essential to centralise
procedures, standardise case definitions, and expand
laboratory capacity to streamline the implementation
of territory-wide surveillance.15
Respiratory syncytial virus
vaccines: efforts for prioritisation
At present, the management of severe RSV infection
is non-specific and largely supportive.2 Although
this approach may discourage testing or screening
in patients presenting with symptomatic infections,
increased quantity and quality of surveillance data
could be used to optimise an RSV vaccination
campaign. For example, such optimisation could
involve prioritisation of high-risk groups, setting
an appropriate age threshold for vaccination,
and determining the overall cost-benefit ratio for
reducing healthcare resource utilisation under
various vaccination coverage scenarios.
To date, two of the three internationally
licensed RSV vaccines are available in Hong Kong:
the adjuvanted RSVPreF3 OA (Arexvy, GSK)
and RSVpreF (Abrysvo, Pfizer) [Table].17 18 19 20 These
vaccines have been evaluated using similar study
designs, although variations exist in the trial centres’
coverage of RSV ‘season’ periods and in the case
definitions used for acute respiratory illness, LRT
illness, and severe LRT illness across at least two RSV
seasons.18 19 20 Both trials have shown high efficacy and
safety of the respective vaccines against symptomatic
infection and severe outcomes (Table).18 20 However,
head-to-head comparisons between the vaccines are
not yet available.
Recent data from the US Centers for Disease
Control and Prevention support the real-world
effectiveness of both RSV vaccines.21 Based on
findings from the VISION multi-site network of
electronic health records (between 1 October
2023 and 31 March 2024), the effectiveness of
the adjuvanted RSVPreF3 OA vaccine against
RSV-associated emergency department visits and hospitalisations was 77% and 83%, respectively,
among adults aged 60 years or above (Table).21
Similarly, RSVpreF demonstrated effectiveness of
79% against emergency department visits and 73%
against hospitalisations.21
The primary concerns regarding RSV
vaccination are similar to those associated with
other vaccines: how to enhance uptake, raise
awareness, address misconceptions, and identify
which populations should be prioritised for free
or subsidised vaccination through public health
programmes.12 15 Unresolved scientific questions—such as the duration of protection and the appropriate
age for vaccine administration—continue to be
investigated and can be informed by local data. The
US Centers for Disease Control and Prevention
recommends RSV vaccination for adults aged 75
years or above, and for those aged 60 to 74 years
with certain chronic medical conditions or other
risk factors (eg, communal living) for severe RSV
infection (Table).22 On 16 April 2025, the Advisory
Committee on Immunization Practices extended
this recommendation to adults aged 50 to 59 years
who are at increased risk of severe RSV infection,
following the US Food and Drug Administration’s
licensure of the vaccine for this population group
(Table).23
Although RSV vaccines are generally well
tolerated, there have been reports of Guillain—Barré syndrome (GBS) and acute disseminated
encephalomyelitis following vaccination.24 25
Assessment of GBS risk after vaccination with
RSVpreF and adjuvanted RSVPreF3 OA was
conducted in a self-controlled case series analysis,
using risk windows defined as 1 to 42 days post-vaccination
and control windows as 43 to 90 days
post-vaccination.25 The analysis of all GBS cases from
this study suggests an increased risk within the first
42 days post-vaccination, equating to seven excess
cases per million doses of adjuvanted RSVPreF3 OA
and nine excess cases per million doses of RSVpreF,
in adults aged 65 years or above.24 25 While the
findings indicate an increased GBS risk, they are
not sufficient to establish a causal relationship.25 In
the RENOIR study, one case each of GBS and Miller
Fisher syndrome (a GBS variant) was reported
after RSVpreF vaccination,19 whereas no cases of
GBS have been reported to date in the AReSVi-006
study investigating the adjuvanted RSVPreF3 OA
vaccine.17 Nonetheless, both vaccines are required to
include a GBS warning, as mandated by the US Food
and Drug Administration.25
Respiratory syncytial virus
vaccination in Hong Kong
Collectively, international experience suggests that
the commercial availability of RSV vaccines will
deliver clinical and public health benefits by reducing severe infections and the utilisation of healthcare resources.
In Hong Kong, the adjuvanted RSVPreF3
OA vaccine is indicated for active immunisation to
prevent LRT disease caused by RSV in adults aged 60
years or above, as well as in adults aged 50 to 59 years
who are at increased risk of RSV disease. RSVpreF
is indicated for active immunisation in individuals
aged 60 years or above to prevent LRT disease caused
by RSV. To promote uptake of privately purchased
(self-paid) vaccines, health education initiatives and
advertising campaigns highlighting the importance
of RSV vaccination should be encouraged.
As of January 2025, due to the lack of cost-benefit
studies in older adults, the Hong Kong
Scientific Committee on Vaccine Preventable
Diseases does not universally recommend RSV
vaccination. Instead, the Committee has advised
that vaccination should be considered, particularly
for individuals aged 75 years or above and those
residing in nursing homes.24 Given that hospitalised
patients with RSV infection frequently present
with comorbidities (over 70% based on available
local data),3 7 it is suggested that vaccination be
prioritised for all adults aged 75 years or above,
immunocompromised individuals, adults aged 60
years or above with relevant comorbid conditions
(eg, chronic obstructive pulmonary disease, asthma,
congestive heart failure, coronary artery disease,
cerebrovascular disease, diabetes mellitus, chronic
kidney disease, or frailty), and those living in
community housing or residential care settings—concordant with recommendations from the
Advisory Committee on Immunization Practices.13
Vaccination subsidies should be considered for at-risk
groups who are economically disadvantaged. The
precise target groups, as well as the potential health
and cost savings from a targeted vaccine rollout, will
depend on local epidemiological data. However, the
development of formal recommendations should be
prioritised by the government and relevant medical
societies involved in the care of at-risk populations.
Conclusion
Respiratory syncytial virus infection is not only a
childhood disease; it also poses a major health risk
to older adults, especially those with underlying
morbidities who require targeted prevention and
treatment. The ageing population in Hong Kong
further exacerbates this challenge. The recent
development of effective vaccines (Table)17 18 19 20 26
underscores the urgent need to develop up-to-date
recommendations and policies to guide
the rational use of vaccination, both to prevent
severe RSV infection and to reduce the associated
healthcare utilisation and societal costs. The precise
determination of target groups should be informed
by local epidemiological data, which can be generated through dedicated studies and enhanced
RSV surveillance, particularly in hospital settings.
In the interim, HCPs are encouraged to proactively
raise awareness of RSV among both medical peers
and the public, and to consider extending vaccination
to at-risk groups in line with international guidance
and published literature. These combined efforts will
promote a coherent policy of systematic vaccination,
achieving the greatest benefit for patients and the
broader community. Future studies should address
the cost-effectiveness of RSV vaccination across
various at-risk populations.12 27
Author contributions
Concept or design: JCK Chan.
Acquisition of data: JCK Chan and MYW Kwan.
Analysis or interpretation of data: All authors.
Drafting of the article: JCK Chan.
Critical revision for important intellectual content: All authors.
Acquisition of data: JCK Chan and MYW Kwan.
Analysis or interpretation of data: All authors.
Drafting of the article: JCK Chan.
Critical revision for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
None of the authors received an honorarium for participating
in the preceding advisory board meeting as declared in the
submitted ICMJE disclosure forms.
Acknowledgement
This editorial incorporates insights from an advisory board
meeting held on 15 July 2024 in Hong Kong, with contributions
from private practitioners Dr Aaron Lai, Dr Leung Cheung
Goh, and Dr Mary Cheng.
Funding/support
Medical writing, editorial, and publication coordination
support were independently funded by GSK in accordance with
the Good Publication Practice (GPP3) guidelines. Editorial
and medical writing support were provided by MediPaper
Medical Communications Ltd. The funders had no role in study
design, data collection, analysis, interpretation, or manuscript
preparation. Ultimate responsibility for the opinions,
interpretation, and conclusions lies with the authors.
References
1. Chan PK, Tam WW, Lee TC, et al. Hospitalization
incidence, mortality, and seasonality of common
respiratory viruses over a period of 15 years in a developed
subtropical city. Medicine (Baltimore) 2015;94:e2024. Crossref
2. Wildenbeest JG, Lowe DM, Standing JF, Butler CC.
Respiratory syncytial virus infections in adults: a narrative
review. Lancet Respir Med 2024;12:822-36. Crossref
3. Lee N, Lui GC, Wong KT, et al. High morbidity and
mortality in adults hospitalized for respiratory syncytial
virus infections. Clin Infect Dis 2013;57:1069-77. Crossref
4. Savic M, Penders Y, Shi T, Branche A, Pirçon JY. Respiratory
syncytial virus disease burden in adults aged 60 years and
older in high-income countries: A systematic literature
review and meta-analysis. Influenza Other Respir Viruses
2023;17:e13031. Crossref
5. Chan WS, Yau SK, To MY, et al. The seasonality of respiratory viruses in a Hong Kong hospital, 2014-2023. Viruses 2023;15:1820.Crossref
6. Wiseman DJ, Thwaites RS, Ritchie AI, et al. Respiratory
syncytial virus-related community chronic obstructive
pulmonary disease exacerbations and novel diagnostics: a
binational prospective cohort study. Am J Respir Crit Care
Med 2024;210:994-1001. Crossref
7. Lui G, Wong CK, Chan M, et al. Host inflammatory
response is the major marker of severe respiratory syncytial
virus infection in older adults. J Infect 2021;83:686-92. Crossref
8. Eden JS, Sikazwe C, Xie R, et al. Off-season RSV epidemics
in Australia after easing of COVID-19 restrictions. Nat
Commun 2022;13:2884. Crossref
9. Centers for Disease Control and Prevention, US
Government. RSV-NET. Respiratory Syncytial Virus
Infection (RSV). 2024. Available from: https://www.cdc.gov/rsv/php/surveillance/rsv-net.html. Accessed 12 Nov 2024.
10. Centre for Health Protection, Department of Health, Hong
Kong SAR Government. Detection of pathogens from
respiratory specimens. Available from: https://www.chp.gov.hk/en/statistics/data/10/641/642/2274.html. Accessed 12 Nov 2024.
11. Pun JC, Tao KP, Yam SL, et al. Respiratory viral infection
patterns in hospitalised children before and after
COVID-19 in Hong Kong. Viruses 2024;16:1786. Crossref
12. Hung IF, Lin AW, Chan JC, et al. Bridging the gap in the
prevention of respiratory syncytial virus infection among
older adults in Hong Kong. Hong Kong Med J 2024;30:196-9. Crossref
13. Britton A, Roper LE, Kotton CN, et al. Use of respiratory
syncytial virus vaccines in adults aged ≥60 years:
updated recommendations of the advisory committee on
immunization practices—United States, 2024. MMWR
Morb Mortal Wkly Rep 2024;73:696-702. Crossref
14. Korean Disease Control and Prevention Agency.
Laboratory surveillance service for influenza and
respiratory viruses. Available from: https://www.kdca.go.kr/contents.es?mid=a30328000000#wrap. Accessed 12 Nov 2024.
15. Bont L, Krone M, Harrington L, et al. Respiratory syncytial
virus: Time for surveillance across all ages, with a focus on
adults. J Glob Health 2024;14:03008. Crossref
16. Teirlinck AC, Broberg EK, Stuwitz Berg A, et al.
Recommendations for respiratory syncytial virus
surveillance at the national level. Eur Respir J
2021;58:2003766. Crossref
17. Papi A, Ison MG, Langley JM, et al. Respiratory syncytial
virus prefusion f protein vaccine in older adults. N Engl J
Med 2023;388:595-608. Crossref
18. Ison MG, Papi A, Athan E, et al. Efficacy and safety of
respiratory syncytial virus (RSV) prefusion F protein
vaccine (RSVPreF3 OA) in older adults over 2 RSV seasons.
Clin Infect Dis 2024;78:1732-44. Crossref
19. Walsh EE, Pérez Marc G, Zareba AM, et al. Efficacy and
safety of a bivalent RSV prefusion F vaccine in older adults.
N Engl J Med 2023;388:1465-77. Crossref
20. Walsh EE, Pérez Marc G, Falsey AR, et al. RENOIR Trial—RSVpreF vaccine efficacy over two seasons. N Engl J Med
2024;391:1459-60. Crossref
21. National Center for Immunization and Respiratory
Diseases, Centers for Disease Control and Prevention, US
Government. Effectiveness of adult respiratory syncytial
virus (RSV) vaccines, 2023-2024. Available from: https://www.cdc.gov/acip/downloads/slides-2024-06-26-28/07-RSV-Adult-Surie-508.pdf. Accessed 29 Apr 2025.
22. Centers for Disease Control and Prevention, US
Government. RSV in older adults. Available from:
https://www.cdc.gov/rsv/older-adults/index.html#:~:text=CDC%20recommends%20everyone%20ages%2075,another%20one%20at%20this%20time . Accessed 12 May 2025.
23. National Center for Immunization and Respiratory
Diseases, Centers for Disease Control and Prevention, US
Government. Evidence to Recommendations Framework
(EtR): RSV vaccination in adults aged 50-59 years. Available
from: https://www.cdc.gov/acip/downloads/slides-2025-04-15-16/06-Melgar-Surie-adult-rsv-508.pdf . Accessed 12 May 2025.
24. Scientific Committee on Vaccine Preventable Diseases,
Centre for Health Protection, Hong Kong SAR
Government. Interim consensus on the use of respiratory
syncytial virus vaccines in Hong Kong (as of 17 January
2025). Available from: https://www.chp.gov.hk/files/pdf/interim_consensus_on_the_use_of_respiratory_syncytial_virus_vaccines_in_hong_kong_jan2025.pdf?f=13 . Accessed 28 Jan 2025.
25. Drug Office, Department of Health, Hong Kong SAR
Government. The United States: FDA requires Guillain-Barré Syndrome (GBS) warning in the prescribing
information for RSV vaccines Abrysvo and Arexvy.
Available from: https://www.drugoffice.gov.hk/eps/news/showNews/The+United+States%3A+FDA+requires+Guillain-Barr%C3%A9+Syndrome+%28GBS%29+warning+in+the+prescribing+information+for+RSV+Vaccines+Abrysvo+and+Arexvy/consumer/2025-01-08/tc/54792.html . Accessed 25 Jan 2025.
26. Ison MG, Papi A, Athan E, et al. Efficacy, safety, and
immunogenicity of the AS01E-adjuvanted respiratory
syncytial virus prefusion F protein vaccine (RSVPreF3
OA) in older adults over three respiratory syncytial
virus seasons (AReSVi-006): a multicentre, randomised,
observer-blinded, placebo-controlled, phase 3 trial. Lancet
Respir Med 2025;13:517-29. Crossref
27. Kwan MY, Chong PC, Chua GT, Ho MH, Poon LC.
Maternal vaccination: a promising preventive strategy
to protect infants from respiratory syncytial virus. Hong
Kong Med J 2024;30:264-7. Crossref

