Hong Kong Med J 2025;31:Epub 29 May 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
EDITORIAL
Establishment of the Institute for Medical Advancement and Clinical Excellence (IMACE)
Gilberto KK Leung, MD, FHKAM (Surgery)1 † Ronald MK Lam , MB, ChB, FHKAM (Community Medicine)2 ‡; Philip WY Chiu, MB, ChB, FHKAM (Surgery)3 ‡; Philip KT Li, DSc, FHKAM (Medicine)1 ‡; William Ho, MB, BS, FHKAM (Community Medicine)4 ‡; Tony PS Ko, MB, BS, FHKAM (Community Medicine)5 ‡;
CS Lau, MD (Dund), FHKAM (Medicine)6 ‡; FC Pang, MB, ChB, FHKAM (Community Medicine)7 ‡; for the Governing Board, Institute for Medical Advancement and Clinical Excellence
1 Hong Kong Academy of Medicine, Hong Kong SAR, China
2 Department of Health, Hong Kong SAR Government, Hong Kong SAR, China
3 Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
4 Hong Kong Private Hospitals Association, Hong Kong SAR, China
5 Hospital Authority, Hong Kong SAR, China
6 Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
7 Primary Healthcare Commission, Health Bureau, Hong Kong SAR Government, Hong Kong SAR, China
Corresponding author: Prof Gilberto KK Leung (gilberto@hku.hk)
† Convenor, Governing Board, Institute for Medical Advancement and Clinical Excellence
‡ Founding Members, Governing Board, Institute for Medical Advancement and Clinical Excellence
Hong Kong’s healthcare system is at a critical
juncture. An ageing population, rising prevalence of
chronic diseases, persistent disparities between the
public and private sectors, and rapid advancements
in medical technology are among the challenges
calling for a new, coordinated, and evidence-based
approach to healthcare service development. The
Government’s initiative to establish the Institute
for Medical Advancement and Clinical Excellence
(IMACE) on 8 May 2025 represents a significant step
forward in addressing these complex issues.1
The IMACE is an independent, professional
platform funded by the Health Bureau, with a
mandate to provide guidance on medical practice
and healthcare delivery. Its formation responds to
several pressing challenges: unwarranted variations
in clinical care, the need for timely evaluation of new
medical technologies, and the imperative to improve
health outcomes across all sectors of our population
in a sustainable manner.
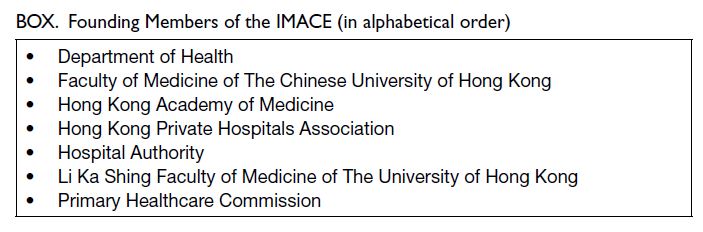
To ensure a diverse coalition that reflects the
views and experiences of different sectors of our healthcare system, seven institutions were invited to
serve as Founding Members (Box). Together with a
Convenor nominated by the Hong Kong Academy
of Medicine, they form the Governing Board that
oversees the institute’s functioning and determines
its strategic directions. A Secretariat housed under
the Hong Kong Academy of Medicine provides
administrative support.
The primary mission of IMACE centres on
developing and disseminating evidence-based
recommendations to guide clinical practice and
healthcare policy. These recommendations,
collectively termed IMACE Recommendations,
may encompass clinical practice guidelines (CPG),
clinical protocols, evaluation of the efficacy and
cost-effectiveness of medical options, and standards
for service quality and efficiency. During the initial
phase we are prioritising the development of CPGs
which may cover, but are not limited to, the use
of medicines, diagnostic technologies, medical
devices, digital technologies, and interventional
procedures in primary, secondary, and community
care. Guideline development will adhere to IMACE’s
Core Principles of being “evidence-based, impartial,
patient-centred, collaborative, and innovative”2 and
follow internationally recognised methodologies
where appropriate, while remaining responsive to
Hong Kong's specific healthcare context.3
A number of factors will be considered by
the Governing Board when identifying clinical
topics for guideline development. These include:
whether there is significant and unwarranted
variation in clinical practice; whether the guideline is likely to reduce avoidable illness, care burden,
significant morbidity and/or premature mortality;
whether the guideline is likely to enhance service
quality and efficiency; whether there is a sufficient
volume of reliable evidence proportionate to the
context of the topic area, and so on.4 To expedite
the guideline development process, reference may
be made to CPGs already published by local or
overseas professional organisations. An operational
framework for prioritisation, implementation, and
quality assurance is under development.
The IMACE CPGs, defined as “systematically
developed statements to assist practitioner and patient
decisions about appropriate health care for specific
clinical circumstances”,5 are applicable to healthcare
professionals and providers across the public and
private sectors in Hong Kong. They are advisory in
nature and intended to support clinical decision-making,
not to replace professional expertise and
individual clinical judgement. The IMACE CPGs are
also intended to facilitate the safe, responsible, and
cost-effective application of new technologies, not to
restrict their adoption. Healthcare professionals and
providers are encouraged to follow IMACE CPGs
where appropriate; any departure from these guidelines
should be supported by good clinical reasons.2
Another focus of our work is public education.
The IMACE recognises that high-quality healthcare
requires informed patients who can actively
participate in their care decisions. However, as
medical practices become increasingly sophisticated,
patients may find it difficult to handle the large
amount of complex information presented by their
doctors or available online. To address this, we plan to
develop patient education materials that explain the
principles of clinical research and medical evidence
in clear, accessible language. These resources aim
to improve health literacy without oversimplifying
information, facilitate constructive doctor-patient
communication, and empower patients to make
informed and personalised decisions.
The IMACE is an unprecedented collaboration,
bringing together institutions that have traditionally
operated independently. Through alignment of shared standards of care while respecting the diversity of our
healthcare landscape, this cooperative model enables
us to address systemic challenges that individual
organisations could not tackle alone. The IMACE is
well-positioned to serve as a catalyst for meaningful
and lasting improvements in Hong Kong’s healthcare
system and, in time, to play an important role in
shaping the future of medicine in the region. The
journey ahead will require the ongoing engagement
of and support from all stakeholders, from frontline
clinicians to hospital administrators, policymakers,
and patients themselves. It is a daunting task, but
also a worthwhile and exciting one.
Author contributions
All authors contributed equally to the conception, preparation,
and editing of the manuscript. All authors approved the final
version for publication and take responsibility for its accuracy
and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
References
1. The Hong Kong SAR Government. Government welcomes
formal establishment of Institute for Medical Advancement
and Clinical Excellence today. 8 May 2025. Available
from: https://www.info.gov.hk/gia/general/202505/08/P2025050800676.htm?fontSize=1. Accessed 8 May 2025.
2. Constitution of the Institute for Medical Advancement
and Clinical Excellence (IMACE). Hong Kong: Institute for
Medical Advancement and Clinical Excellence; 2025.
3. National Institute for Health and Care Excellence.
Developing NICE guidelines: the manual. NICE process
and methods. 31 October 2014 (last updated 29 May 2024).
Available from: https://www.nice.org.uk/process/pmg20/chapter/introduction. Accessed 1 May 2025.
4. National Institute for Health and Care Excellence. NICEwide
topic prioritisation: the manual. NICE process and
methods. 29 May 2024 (last updated 31 March 2025).
Available from: https://www.nice.org.uk/process/pmg46.
Accessed 1 May 2025.
5. Institute of Medicine, Committee to Advise the Public
Health Service on Clinical Practice Guidelines. Clinical
Practice Guidelines: Directions for a New Program. US:
Washington DC, National Academies Press; 1990.