Hong Kong Med J 2025;31:Epub 6 Oct 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Endovascular management of stent graft dislodgement during thoracic endovascular aortic repair: a case report
Cheng Zeng1 #, Qiulong Min2 #, Rong Ye1, Zhibiao Le1, Yi Li2, Xunhong Duan1, Qing Duan1, Fengen Liu1
1 Department of Vascular Surgery, First Affiliated Hospital of Gannan Medical University, Ganzhou, China
2 The First Clinical Medical School of Gannan Medical University, Ganzhou, China
# Equal contribution
Corresponding author: Mr Fengen Liu (Liufengen9356@163.com)
Case presentation
In November 2023, a 47-year-old male was
admitted to our hospital for surgical management
of an aneurysm in the proximal segment of the
descending thoracic aorta (Fig 1). The patient
reported no prior relevant medical interventions.
Thoracic endovascular aortic repair (TEVAR) was
planned. A left common carotid-to-left subclavian bypass, followed by covered stent-graft placement in
the descending aorta, was scheduled to extend the
proximal landing zone and prevent stent collapse
into the aneurysm.
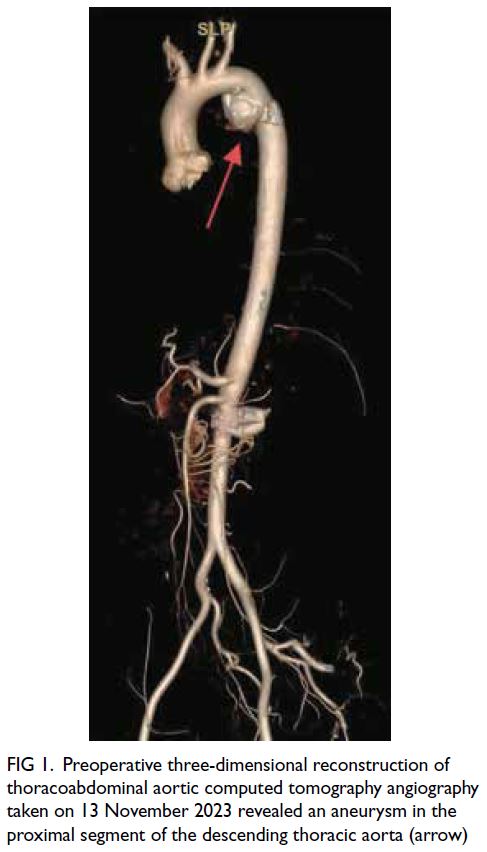
Figure 1. Preoperative three-dimensional reconstruction of thoracoabdominal aortic computed tomography angiography taken on 13 November 2023 revealed an aneurysm in the proximal segment of the descending thoracic aorta (arrow)
A covered stent graft (28 mm diameter,
150 mm long) [TAG; Gore Medical, Flagstaff
[AZ], US] was deployed. Nonetheless, post-deployment
angiography revealed that the stent
had migrated proximally, unintentionally covering
the left common carotid artery. To resolve this
complication, a chimney stent was planned for
the left common carotid artery. A bare metal stent
(10 mm diameter, 40 mm long) [Wallstent; Boston
Scientific, Marlborough [MA], US] was deployed
but subsequently dislodged and migrated into the
ascending aorta due to preoperative underestimation
of the vessel diameter and anterior jumping of the
stent during deployment (Fig 2a).
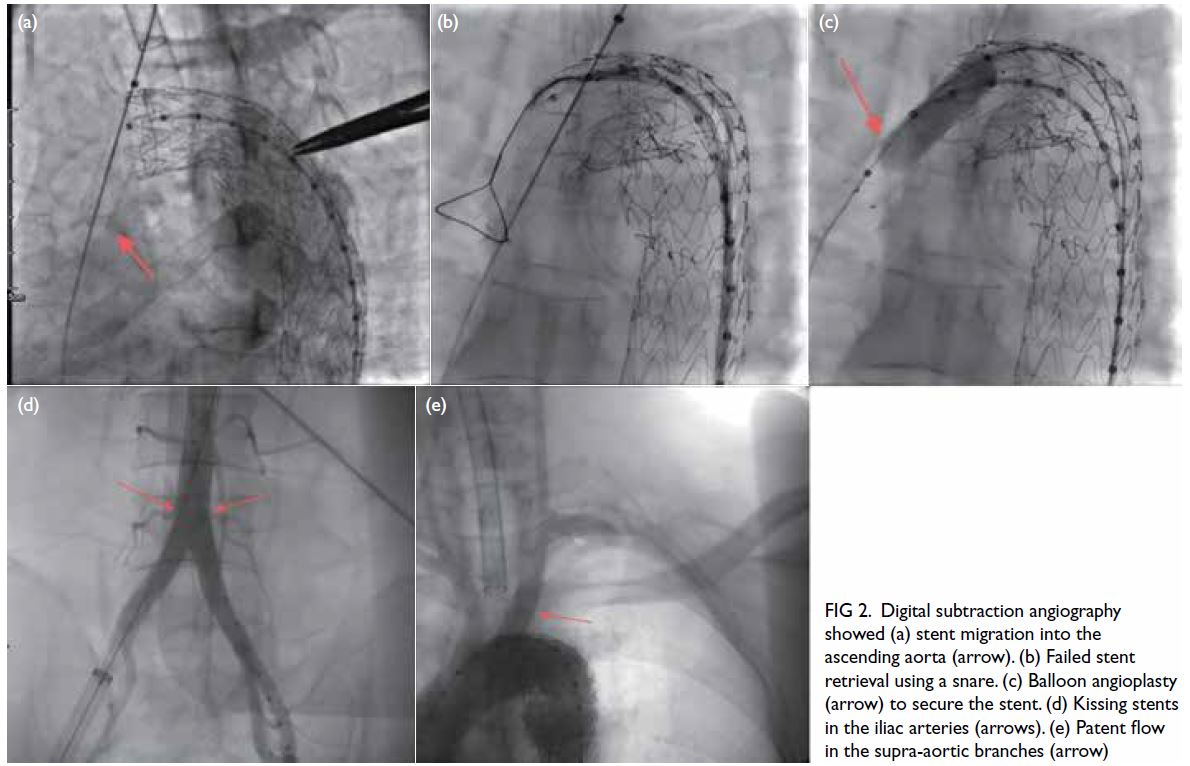
Figure 2. Digital subtraction angiography showed (a) stent migration into the ascending aorta (arrow). (b) Failed stent retrieval using a snare. (c) Balloon angioplasty (arrow) to secure the stent. (d) Kissing stents in the iliac arteries (arrows). (e) Patent flow in the supra-aortic branches (arrow)
Initial attempts to retrieve the stent with a
snare catheter and drag it to the femoral artery
were unsuccessful (Fig 2b). Subsequently, a single-curved
catheter and hydrophilic guidewire were
used to selectively cannulate the migrated stent. A
balloon catheter was then advanced into the stent
and inflated to secure firm attachment, allowing
successful traction of the stent into the right iliac
artery (Fig 2c).
To restore cerebral perfusion, a second balloon-expandable
stent (10 mm × 40 mm) [Express; Boston
Scientific, Marlborough [MA], US] was deployed
in the left common carotid artery to complete the
chimney stent placement. To prevent contralateral
flow obstruction and acute ischaemia in the left
lower limb due to the right iliac stent, an identical
stent was placed via the left femoral artery, creating
a kissing stent configuration in the iliac arteries (Fig 2d). Final angiography confirmed the absence of any
endoleak and demonstrated patent blood flow in the
aortic arch branch vessels and both iliac arteries (Fig 2e).
The patient improved and was discharged
1 week postoperatively. Follow-up computed
tomography angiography of the thoracoabdominal aorta at 6 days and 6 months postoperatively revealed
proper positioning of the stents, with unobstructed
blood flow within the stented vessels (Fig 3).
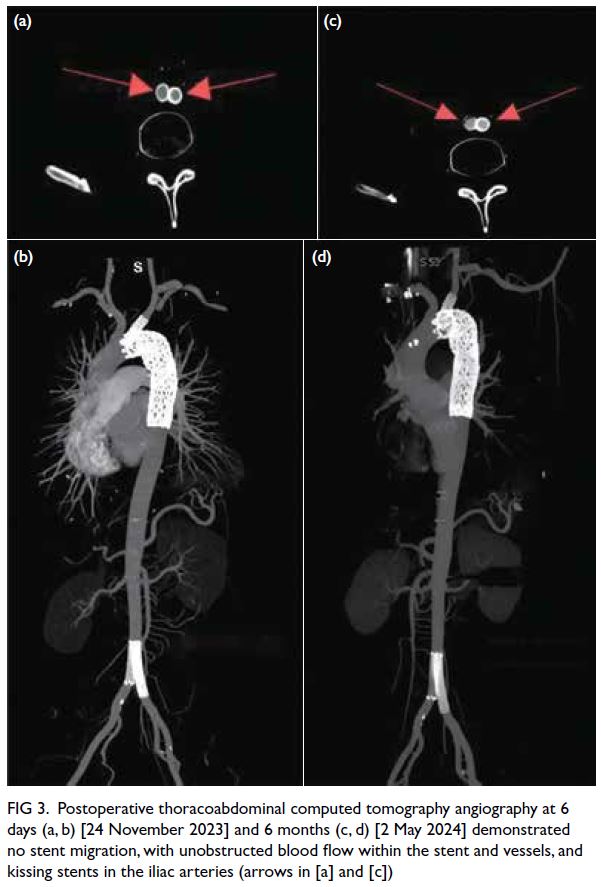
Figure 3. Postoperative thoracoabdominal computed tomography angiography at 6 days (a, b) [24 November 2023] and 6 months (c, d) [2 May 2024] demonstrated no stent migration, with unobstructed blood flow within the stent and vessels, and kissing stents in the iliac arteries (arrows in [a] and [c])
Discussion
Thoracic aortic aneurysm (TAA) is a severe vascular
disease characterised by abnormal dilation of the
thoracic segment of the aorta. Between 1999 and
2020, 47 136 adults in the United States died from
TAA.1 The age-adjusted mortality rate significantly
decreased from 16.2 per million in 1999 to 8.2 per
million in 2013 (annual percentage change: -5.00,
95% confidence interval [95% CI]= -5.54 to -4.54;
P<0.001),1 highlighting the impact of targeted
interventions.
Conventional management of TAA focuses
on controlling blood pressure and heart rate, with
elective surgery for eligible patients. Thoracic
endovascular aortic repair has emerged as an
effective, minimally invasive treatment. A previous
study demonstrated that TEVAR significantly
reduced 30-day all-cause mortality (odds ratio=0.44,
95% CI=0.33-0.59) and paraplegia (odds ratio=0.42,
95% CI=0.28-0.63) compared with open repair.2 The procedure also lowers the risk of cardiac
complications, transfusion need, reoperation for
bleeding, renal insufficiency, and pneumonia, with
a shorter hospital stay. Nonetheless, no significant
differences between the two approaches have been
reported for rates of stroke, myocardial infarction,
aortic reintervention, or 1-year mortality.
Another study found higher early postoperative
mortality with open repair but improved long-term
survival.3 Despite these advantages, TEVAR showed
a higher overall mean survival rate, making it a strong
contender as a first-line treatment for descending
TAA. For patients with multiple co-morbidities or
poor overall health, traditional open surgery carries
excessive risks, further cementing TEVAR as the
preferred option for this group.
When TAA is located near the supra-aortic
branches, standalone TEVAR may be suboptimal.
In such cases, supra-aortic branch reconstruction
or bypass is necessary to extend the proximal
landing zone, reducing the risk of endoleak and
stent collapse into the aneurysm, and preventing
catastrophic outcomes. Hybrid procedures,
which combine open surgery with endovascular
techniques, have emerged as a promising option for high-risk patients. Nonetheless, even hybrid repair
for thoracoabdominal aortic pathology carries
significant morbidity and mortality for patients
deemed unfit for conventional surgery.4 As a result, lifelong regular follow-up is crucial for assessing the
long-term performance of the graft.
Thoracic endovascular aortic repair and its
graft-related complications—such as endoleak,
stent fracture, and migration—can lead to aneurysm
expansion, rupture, and the need for reintervention.
A retrospective analysis of 123 patients treated with
TEVAR for TAA, aortic dissection, penetrating aortic
ulcer, intramural haematoma, or traumatic rupture
revealed a stent stability rate of 99.1% at 1 year, 94.0%
at 3 years, and 86.1% at 5 years.5 Thoracic aortic aneurysm and aortic elongation were identified as
key risk factors for stent migration.
Thoracic stent-graft migration is a common
complication of TEVAR. Intraoperative dislodgement
of branch stents into the ascending aorta is a rare but
life-threatening event. In this case, the left common
carotid artery stent dislodged into the ascending
aorta during the procedure. The conventional
response is to convert to open surgery to retrieve the
stent, but this increases surgical time and complexity
and may be challenging in emergencies. Delayed
revascularisation can lead to cerebral infarction or
neurological impairment.
Accurate preoperative assessment of vessel
diameter, appropriate oversizing, and meticulous
intraoperative technique can effectively reduce
the risk of stent dislodgement. In this case, a
balloon catheter was used to pull the dislodged
stent into the right iliac artery, followed by prompt
revascularisation of the left common carotid artery,
thereby minimising neurological risk. A stent was
placed in the left iliac artery to prevent contralateral
limb ischaemia. Intraoperative digital subtraction
angiography and postoperative computed
tomography angiography confirmed proper stent
positioning and patency of the graft vessels.
This case demonstrates that the use of a
balloon catheter to retrieve dislodged branch stents
to a distal location facilitates effective endovascular
management. With meticulous intraoperative
monitoring, minimally invasive techniques can
address complex complications, avoiding the
risks associated with open surgery. This approach
provides a novel endovascular strategy for managing
branch stent dislodgement.
Author contributions
Concept or design: C Zeng, Q Min.
Acquisition of data: R Ye, Y Li.
Analysis or interpretation of data: C Zeng, Q Min, Z Le.
Drafting of the manuscript: X Duan, Q Duan.
Critical revision of the manuscript for important intellectual content: F Liu.
Acquisition of data: R Ye, Y Li.
Analysis or interpretation of data: C Zeng, Q Min, Z Le.
Drafting of the manuscript: X Duan, Q Duan.
Critical revision of the manuscript for important intellectual content: F Liu.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration
of Helsinki and provided written informed consent for all
treatments and procedures, as well as for publication of this
case report and the accompanying clinical images.
References
1. Goyal A, Saeed H, Shamim U, et al. Trends and disparities
in age, sex, ethnoracial background, and urbanization
status in adult mortality due to thoracic aortic aneurysm:
a retrospective nationwide study in the United States. Int J
Surg 2024;110:7647-55. Crossref
2. Cheng D, Martin J, Shennib H, et al. Endovascular aortic
repair versus open surgical repair for descending thoracic
aortic disease: a systematic review and meta-analysis of
comparative studies. J Am Coll Cardiol 2010;55:986-1001. Crossref
3. Chiu P, Goldstone AB, Schaffer JM, et al. Endovascular
versus open repair of intact descending thoracic aortic
aneurysms. J Am Coll Cardiol 2019;73:643-51. Crossref
4. Moulakakis KG, Mylonas SN, Avgerinos ED, Kakisis JD,
Brunkwall J, Liapis CD. Hybrid open endovascular
technique for aortic thoracoabdominal pathologies.
Circulation 2011;124:2670-80. Crossref
5. Geisbüsch P, Skrypnik D, Ante M, et al. Endograft
migration after thoracic endovascular aortic repair. J Vasc
Surg 2019;69:1387-94. Crossref

