Hong Kong Med J 2025;31:Epub 17 Jul 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
D-lactic acidosis in short bowel syndrome: are
probiotics friend or foe? A case report
Bowie PY Leung, MRCPCH, FHKAM (Paediatrics)1; Bess SY Tsui, FHKAM (Surgery), FCSHK2; Ingrid Kan, MSc (Nutrition and Dietetics), APD3
1 Department of Paediatrics, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong SAR, China
2 Department of Surgery, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong SAR, China
3 Department of Dietetics, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong SAR, China
Corresponding author: Dr Bowie PY Leung (bowieleung@cuhk.edu.hk)
Case presentation
A 6-year-old Chinese boy with short bowel syndrome
(SBS) presented to the emergency department with
excessive drowsiness. He was born full term with an
unremarkable perinatal history and had good past
health. At 3 years of age, he underwent extensive
small bowel resection and a right hemicolectomy
due to intestinal malrotation with midgut volvulus,
resulting in a residual length of 66 cm of proximal
small bowel and distal colon, with loss of the
ileocaecal valve.
Initially dependent on total parenteral
nutrition, he achieved enteral autonomy 3 years
later, consuming an oral diet supplemented
with vitamins, iron, and a hydrolysed formula of
1 kcal/mL, contributing approximately 20% of total energy intake. A timeline summarising key clinical
events, including enteral and parenteral nutrition
milestones, is presented in the Figure.
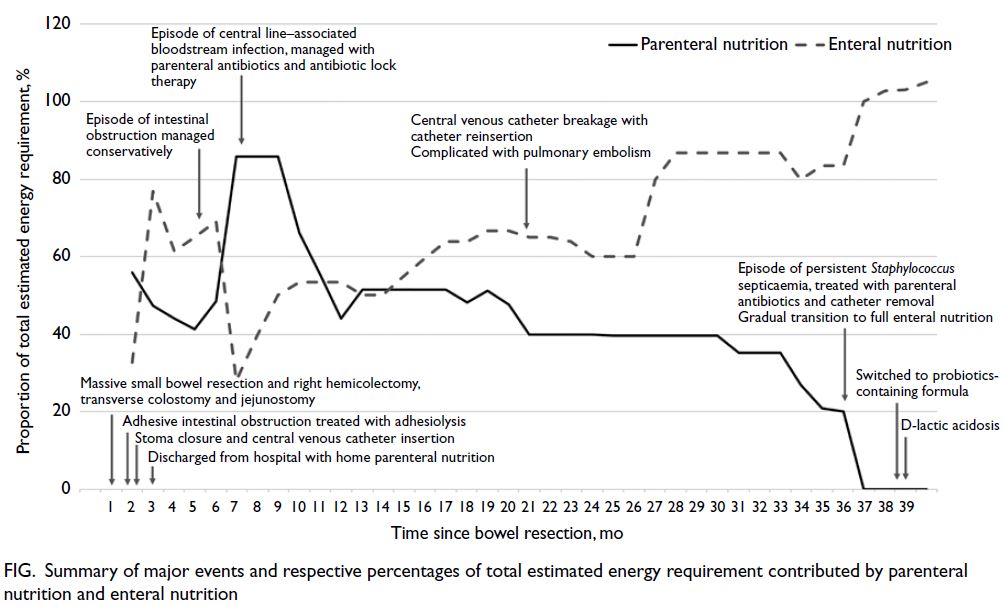
Figure. Summary of major events and respective percentages of total estimated energy requirement contributed by parenteral nutrition and enteral nutrition
He was reviewed monthly by a multidisciplinary
team with regular assessments of his nutritional
status, growth parameters, and biochemical profile.
He demonstrated good growth, maintaining weight
and height at the 50th percentile, with regular
bowel movements with daily oral loperamide. His
biochemical profile, including blood counts, liver
function, electrolytes, blood gas, and trace elements,
remained stable throughout the follow-up period.
On this admission, the patient was drowsy and
lethargic but not confused. Blood tests indicated
high anion gap metabolic acidosis, with a pH of
7.31, bicarbonate 10.8 mmol/L, pCO2 2.9 kPa, and L-lactate 1.6 mmol/L (reference range, 1.0-2.4
mmol/L). Complete blood counts, liver enzymes,
ammonia, electrolytes, glucose levels, and computed
tomography of the brain were normal. D-lactic
acidosis (D-LA) was confirmed by an elevated serum
D-lactate concentration of 1.7 mmol/L (normal
range, <0.5 mmol/L). Further enquiry revealed
that one week prior, his family had switched to an
alternative commercially available enteral formula
containing probiotics (Lactobacillus paracasei and
Bifidobacterium longum) as the original formula
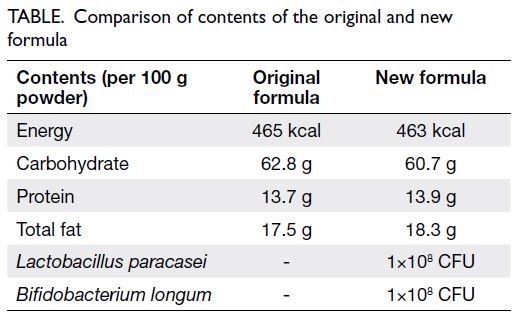
was temporarily out of stock (Table). The rest of his
oral diet remained unchanged. Total carbohydrate
(CHO) intake accounted for 40% to 50% of his total
enteral intake, with the formula contributing 20%.
His condition improved rapidly with bowel
rest and oral sodium bicarbonate. He was treated
with a course of oral metronidazole. The probiotic-containing
formula was stopped, and he was
instructed to resume the original probiotic-free
hydrolysed formula along with CHO-restricted
meals. His carers were re-educated on CHO counting
and avoidance of simple sugars. He remained
clinically stable the following months, during which
he maintained good dietary compliance.
Discussion
Short bowel syndrome refers to a condition of
intestinal malabsorption resulting from loss or
surgical resection of the small intestine and is the
leading cause of intestinal failure. It encompasses
a heterogeneous group of patients with various
aetiologies and bowel anatomies. Effective
management requires a multidisciplinary approach
to promote enteral autonomy, support growth, and
prevent complications such as catheter-related
bloodstream infections and intestinal failure–associated liver disease.
D-lactic acidosis, first described in SBS by
Oh et al in 1979,1 has gained increasing recognition
as a rare but serious metabolic complication.
It results from intestinal malabsorption and
overgrowth of colonic microbiota (eg, Lactobacillus spp, Bifidobacterium spp), leading to excessive
fermentation of unabsorbed CHO. The process
is exacerbated by factors such as high CHO
intake, elevated gut pH, impaired gut motility,
antimicrobials, probiotics, and intestinal infections.
The overproduction of D-lactic acid leads to a
neurological syndrome and high anion gap metabolic
acidosis. Clinical manifestations include acidotic
breathing, altered mental state, ataxia, slurred
speech, nystagmus, gait disturbance, behavioural
change, and fatigue. A high index of clinical suspicion
and measurement of D-lactic acid are essential for
diagnosis, as serum lactate concentration (reflecting
L-lactate) is often normal.2 3
The mainstays of acute management of D-LA
include correction of metabolic acidosis with
bicarbonate and rehydration, restriction of enteral
CHO intake, administration of poorly absorbed
oral antibiotics, and avoidance of antimotility
agents or lactate-containing solutions. Additional
treatment may include thiamine and riboflavin
supplementation, insulin, and short-chain fatty
acids. Metabolic acidosis and neurological
symptoms often improve rapidly with early and
appropriate intervention. To prevent recurrence,
CHO restriction and avoidance of D-lactate–containing foods (eg, pickles and yoghurt) are
essential. In selected cases, suppression of abnormal
gut flora with antimicrobials or surgery to increase
bowel absorptive area may be considered.3 4
Probiotics have gained popularity as health-promoting
agents in medicines and dietary
supplements, including in the management
of SBS to prevent and treat small intestinal
bacterial overgrowth. Certain species, such as
Lactobacillus casei, produce only L-lactate. Among
commercially available probiotics, Lactobacillus
and Bifidobacterium are the most commonly used
genera.2 5 The European Society for Paediatric
Gastroenterology, Hepatology and Nutrition has
summarised the latest evidence on probiotic use
across various paediatric gastrointestinal disorders.6
Strain-specific benefits have been demonstrated in
conditions such as acute gastroenteritis, antibiotic-associated
diarrhoea, infantile colic, functional
abdominal disorders, and in the prevention of
necrotising enterocolitis and nosocomial diarrhoea.6
Animal studies and clinical case reports
suggest that probiotics may confer potential benefits
in patients with SBS through mechanisms such as
enhancement of gut barrier function, suppression of
pathogens, and modulation of immune responses.7
Nevertheless, clinical studies evaluating their efficacy
remain limited, and there is insufficient evidence
to support the routine use in SBS. Conversely, case
reports have raised safety concerns, such as the
development of D-LA and sepsis in children with
SBS following probiotic administration.7 In our case, the addition of probiotics via the new milk
formula suggests a possible role of probiotics in
the development of D-LA. This highlights the need
for cautious and selective use of non–D-lactate–producing probiotic strains in patients at high risk of
D-LA.
This report illustrates a case of D-LA in a
paediatric patient with SBS, precipitated by the intake
of a probiotic-containing enteral formula. Early
recognition of D-LA, based on characteristic clinical
features and confirmed by D-lactate measurement,
with prompt treatment to normalise acidosis and
suppress D-lactate production, is essential. Cautious
dietary management, including caregiver awareness
of formula contents and dietary CHO restriction, is
equally important. Despite the increasing medical
use of probiotics, there is a lack of clinical trials to
support their routine use or provide clear guidance
for their use in paediatric SBS. Careful consideration
is warranted, with awareness of potential strain-specific
benefits and risks, particularly in patients
with altered intestinal microbiota and malabsorption.
Author contributions
Concept or design: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: BPY Leung.
Critical revision for important intellectual content: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: BPY Leung.
Critical revision for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors would like to acknowledge the multidisciplinary
teams involved in the co-management of this case. Special
thanks to the dietitians, Paediatric Surgery team, and
Paediatric Gastroenterology team at Prince of Wales Hospital,
as well as the Paediatric team at Princess Margaret Hospital, for their invaluable contributions to the care and management
of the patient.
Declaration
Findings from this case were presented as a poster at the 23rd
Congress of the Parenteral and Enteral Nutrition Society of
Asia (PENSA 2023), 19-22 October 2023, Taipei, Taiwan.
Funding/support
This case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration
of Helsinki and provided written informed consent for all
treatments, procedures and the publication of this case report.
References
1. Oh MS, Phelps KR, Traube M, Barbosa-Saldivar JL, Boxhill C, Carroll HJ. D-lactic acidosis in a man with the short-bowel syndrome. N Engl J Med 1979;301:249-52. Crossref
2. Muto M, Kaji T, Onishi S, Yano K, Yamada W, Ieiri S.
An overview of the current management of short-bowel
syndrome in pediatric patients. Surg Today 2022;52:12-21. Crossref
3. Bianchetti DG, Amelio GS, Lava SA, et al. D-lactic acidosis
in humans: systematic literature review. Pediatr Nephrol
2018;33:673-81. Crossref
4. Kowlgi NG, Chhabra L. D-lactic acidosis: an
underrecognized complication of short bowel syndrome.
Gastroenterol Res Pract 2015;2015:476215. Crossref
5. Höllwarth ME, Solari V. Nutritional and pharmacological
strategy in children with short bowel syndrome. Pediatr
Surg Int 2021;37:1-15. Crossref
6. Szajewska H, Berni Canani R, Domellöf M, et al. Probiotics
for the management of pediatric gastrointestinal disorders:
position paper of the ESPGHAN Special Interest Group on
Gut Microbiota and Modifications. J Pediatr Gastroenterol
Nutr 2023;76:232-47. Crossref
7. Reddy VS, Patole SK, Rao S. Role of probiotics in short
bowel syndrome in infants and children—a systematic
review. Nutrients 2013;5:679-99. Crossref