Hong Kong Med J 2025;31:Epub 6 Aug 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Clinical outcomes after implementation of a lung
nodule surveillance programme in Hong Kong
Lynn YW Shong, PhD, FHKAM (Medicine)1; WC Chong, MScEPB, MN2; PI Cheang, BNurs1; WC Choy, MSc2; Florence KP Chan, MD, FHKAM (Medicine)2; WC Kwok, MD, FHKAM (Medicine)2; Mary SM Ip, MD, FRCP (UK)2; David CL Lam, MD, PhD2
1 Division of Respiratory Medicine, Department of Medicine, Queen Mary Hospital, Hong Kong SAR, China
2 Division of Respiratory Medicine, Department of Medicine, School of
Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of
Hong Kong, Hong Kong SAR, China
Corresponding author: Prof David CL Lam (dcllam@hku.hk)
Abstract
Introduction: To facilitate early and accurate
identification and risk stratification of lung nodules,
a surveillance programme was implemented at a
tertiary hospital in Hong Kong. This study examined
the clinical outcomes of patients recruited during
the first year of programme implementation.
Methods: This prospective cohort study included
patients enrolled in the lung nodule surveillance
programme between 1 January 2022 and 31 December
2022. Recruitment criteria included patients
attending the respiratory outpatient clinic for lung
nodules or lung masses. Patient demographics and
clinical outcomes were analysed. Primary outcomes
were the number of lung cancer cases detected
and their stage at diagnosis. Secondary outcomes
included the invasive investigations performed,
adverse events related to these procedures, and
details of lung cancer treatment and survival.
Results: Of the 1471 patients recruited to the
programme, 291 (19.8%) underwent invasive
investigations, and 133 (9.0%) were diagnosed
with lung cancer. Among those diagnosed, 62
(46.6%) had stage I disease and 10 (7.5%) had stage
II disease. Overdue scans and missed follow-ups
were identified and rescheduled. Significantly better
survival was observed in female patients compared
with male patients (P=0.037 for progression-free survival and P=0.030 for overall survival), and in
patients with early-stage cancer compared with
those with late-stage lung cancer (P<0.001). Age
was also independently associated with survival
outcomes (P<0.001).
Conclusion: The implementation of a lung nodule
surveillance programme resulted in the detection
of early-stage lung cancer in more than half of
diagnosed cases, with the potential to improve
patient survival.
New knowledge added by this study
- The implementation of a lung nodule surveillance programme improved compliance with follow-up. The proportion of early-stage lung cancer diagnoses in this programme was higher than that reported by the Hong Kong Cancer Registry in 2021.
- Patients with early-stage lung cancer and female patients demonstrated better survival outcomes.
- This lung nodule surveillance programme has the potential to improve lung cancer survival rates and reduce healthcare costs.
- Further research on the cost-effectiveness of such programme is essential to inform healthcare policy and optimise care for patients with lung nodules.
Introduction
Lung cancer is the leading cause of cancer-related
deaths in Hong Kong, with a high incidence
and low 5-year survival rate.1 Early diagnosis
and treatment are crucial to improving survival
outcomes, as demonstrated in lung cancer
screening trials and real-world experiences.2 3 4 5
To date, low-dose computed tomography (CT) screening has not been implemented in Hong
Kong.6 However, the widespread clinical use of CT
has led to increased detection of lung nodules on
thoracic CT scans.7 Despite general awareness of
lung nodule management guidelines, adherence
is often suboptimal.8 9 Over the past few decades,
an increasing number of centres have developed
programmes to manage incidental lung nodules.10 This study aimed to review the clinical outcomes of
a lung nodule surveillance programme at a tertiary
hospital in Hong Kong. It outlines the structure
of this comprehensive programme, characteristics
of lung nodules, invasive interventions, stage
distribution, treatment modalities, and initial
survival data.
Methods
Participants
This single-centre prospective cohort study included
all patients enrolled in a lung nodule surveillance
programme at Queen Mary Hospital, Hong Kong,
during its first year of implementation, from 1 January
to 31 December 2022. The programme was launched
with the following objectives: (1) to streamline the
referral process and promote cohesive care among
practitioners; (2) to identify high-risk lung nodules
requiring timely invasive intervention; (3) to provide
a multidisciplinary platform with a nurse navigator
coordinating care across specialties; and (4) to
establish rapport between patients and healthcare
staff, thereby improving compliance. Recruitment
criteria included patients under surveillance for lung
nodules (lesions <3 cm) or lung masses. Patients with
lung cancer receiving active treatment were excluded.
Referral sources included lung cancer screening,
incidental findings on CT or chest radiographs, and
symptomatic presentations. All recruited cases were
followed until the lung nodule was confirmed as benign or malignant, or until participants declined
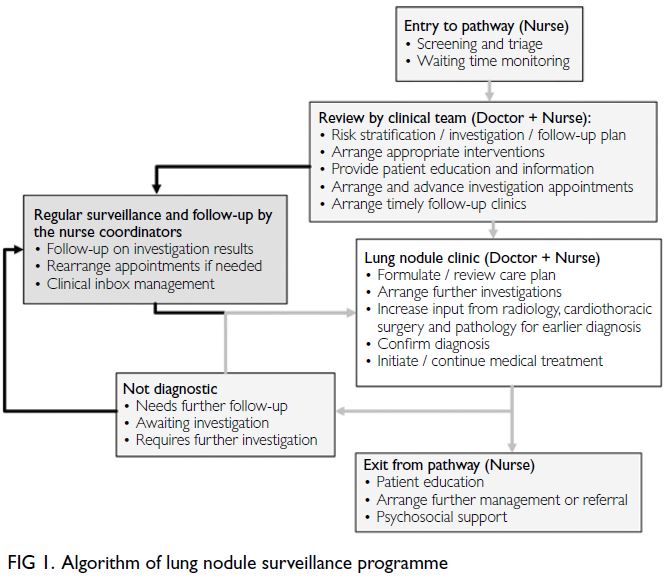
follow-up or died. The programme algorithm is
shown in Figure 1. Upon entry into the pathway, a
nurse coordinator conducts initial screening and
monitors patients’ waiting time. The clinical team,
comprising doctors and nurses, reviews each case to
perform risk stratification, formulate investigation
and follow-up plans, arrange appropriate
interventions, provide patient education, and ensure
timely follow-up. Patients are managed according
to the 2017 Fleischner Society Guidelines11 and the
Clinical Practice Consensus Guidelines for Asia.12
Lung nodules requiring intensive evaluation or
tissue sampling are discussed by a multidisciplinary
team that includes respiratory physicians, chest
radiologists, cardiothoracic surgeons, and clinical
oncologists. Nurse coordinators regularly review
consultation notes and track investigation results.
Overdue scans or missed follow-ups are identified
and rescheduled. Surveillance continues until the
lung nodule is confirmed as benign or malignant.
The demographic and clinical characteristics of
all recruited individuals including sex, age, smoking
history, and co-morbidities were retrieved from
the Clinical Management System of the Hospital
Authority. In pathology reports, cases where non–small-cell lung cancer (NSCLC) could not be further
classified were recorded as NSCLC. In the results,
adenocarcinoma, squamous cell carcinoma, smallcell
lung cancer, and NSCLC were listed as mutually
exclusive categories. Cancer staging was assigned
based on pathological staging when available, or
clinical staging otherwise, in accordance with the
eighth edition of the International Association for
the Study of Lung Cancer staging classification.13
Patients with stage I or II lung cancer were categorised
as early-stage, and those with stage III or IV disease
as late-stage. The STROBE (Strengthening the
Reporting of Observational Studies in Epidemiology)
reporting guideline was followed in the preparation
of this manuscript. Primary outcomes were the
proportion of lung cancer cases detected and their
stage at diagnosis. Secondary outcomes included
invasive investigations performed, adverse events
related to invasive procedures, subgroup analysis
by referral source, avoidance of overdue scans and
loss to follow-up, and lung cancer treatment and
survival.
Statistical analyses
Continuous variables were reported as mean with
standard deviation or median with interquartile
range, as appropriate. Categorical variables were
reported as number and percentage. Clinical
characteristics between subgroups were analysed
using Student’s t test or Chi squared test. Progression-free
survival (PFS) and overall survival (OS) were
analysed using the Kaplan–Meier method, and factors affecting survival were evaluated using Cox
regression analysis. All analyses were performed
using SPSS (Windows version 23.0; IBM Corp,
Armonk [NY], US).
Results
Study population
A total of 1471 adult patients with lung nodules or lung
masses were enrolled in the lung nodule surveillance
programme. Demographic characteristics are
summarised in online supplementary Table 1. The
mean age was 68 years; 726 (49.4%) were men, 954
(64.9%) were never-smokers, 150 (10.2%) were
current smokers, and 367 (24.9%) were ex-smokers.
Regarding co-morbidities, 140 patients (9.5%) had
chronic obstructive pulmonary disease, 35 (2.4%)
had interstitial lung disease, 59 (4.0%) had asthma,
and 105 (7.1%) had a history of tuberculosis. Of the
1471 participants, 291 (19.8%) underwent invasive
diagnostic procedures, resulting in the diagnosis
of lung cancer in 133 patients (9.0%), including
131 primary lung cancers and two cases of lung
metastasis from pancreatic carcinoma (online supplementary Fig 1). Pathological findings of the 158 patients without lung cancer are presented in
online supplementary Table 2. Referral sources and
radiographic characteristics of 266 patients, including
133 with lung cancer and 133 matched controls
(matched by age, sex, and smoking history) without
lung cancer, are presented in online supplementary Table 3. Among these 266 patients, 193 (72.6%) had
more than one pulmonary lesion, 67.3% of dominant
nodules were solid, and 50.0% were located in
the upper lobes. The median size of the dominant
lung lesion was 12 mm (interquartile range, 6-26).
Patients with lung cancer had significantly larger
pulmonary lesions (median size: 24 mm vs 6 mm,
P<0.001) and a higher proportion of part-solid
nodules (21.1% vs 3.0%, P<0.001) than those without
lung cancer. Referral sources among the 266 patients
included lung cancer screening (29/266, 10.9%),
incidental findings on CT or chest radiographs
(153/266, 57.5%), and symptomatic presentation
(84/266, 31.6%). There were no significant differences
between groups in referral source, nodule number,
or location. Among the 133 patients without lung
cancer, 31 (23.3%) missed scheduled follow-up and
12 (9.0%) had overdue scans. All were identified
by programme coordinators and rescheduled for
follow-up.
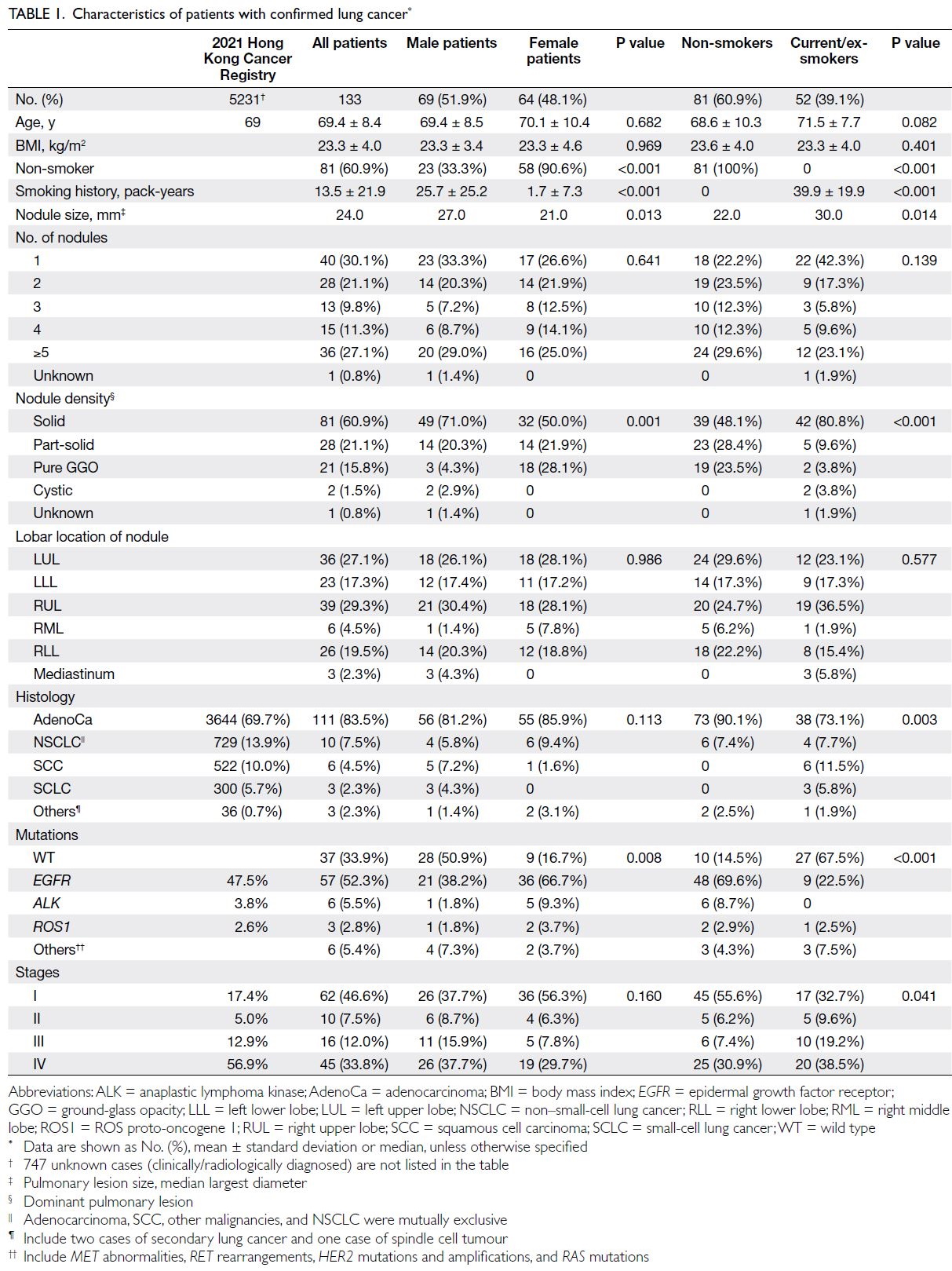
Characteristics of patients with confirmed
lung cancer
The characteristics of patients with confirmed lung
cancer (n=133), both overall and stratified by sex
(men: n=69; women: n=64) and smoking history
(non-smokers: n=81; current/ex-smokers: n=52), are presented in Table 1. The mean age of patients
with lung cancer was 69.4 years; 51.9% were men and
60.9% were non-smokers. There were no significant
differences between groups in terms of age or body
mass index. Compared with male patients, female
patients had significantly lower cigarette exposure
(mean pack-years: 1.7 vs 25.7, P<0.001) and a higher
prevalence of epidermal growth factor receptor
(EGFR) mutations (66.7% vs 38.2%, P=0.008).
Compared with current or ex-smokers, non-smokers
were more likely to have adenocarcinoma (90.1% vs
73.1%, P=0.003), EGFR mutations (69.6% vs 22.5%,
P<0.001), and stage I lung cancer (55.6% vs 32.7%,
P=0.041).
Of the 133 patients with lung cancer, 92 (69.2%)
had more than one pulmonary lesion. Among the
dominant lesions, 60.9% were solid and 56.4% were
located in the upper lobes. The median size of the
dominant lesion was 24.0 mm (interquartile range,
16.0-39.3), and 48 (36.1%) cases presented with lung
masses. Male patients and individuals with a history
of smoking had significantly larger pulmonary
lesions (mean size: 37.2 mm vs 26.1 mm for men and
women, P=0.013; 38.8 mm vs 27.5 mm for current
or ex-smokers and non-smokers, P=0.014). Female
patients exhibited a higher prevalence of ground-glass
opacities (GGOs) compared with male patients
(28.1% vs 4.3%, P=0.002) and a lower proportion of
solid lesions (50.0% vs 71.0%, P=0.004). Non-smokers
were more likely to have pure GGOs (23.5% vs 3.8%,
P=0.004) and part-solid GGOs (28.4% vs 9.6%,
P=0.016) but fewer solid lesions (48.1% vs 80.8%,
P<0.001), compared with current or ex-smokers.
Diagnostic investigations
Among the 291 patients who underwent
invasive investigations, two experienced a small
pneumothorax following transbronchial biopsy
during bronchoscopy, and five developed a minor
pneumothorax after CT-guided biopsy of a lung
lesion; all resolved with conservative management.
Another two patients had a large pneumothorax
following CT-guided biopsy of a lung nodule,
requiring chest drain insertion, which was removed
after 3 days. One patient developed trace perilesional
haemorrhage after CT-guided biopsy of a lung lesion,
which was managed conservatively. No patients
who underwent invasive procedures experienced
complications such as haemothorax, respiratory
failure requiring mechanical ventilation, or death.
The diagnoses for the 133 patients with lung cancer
were established using various diagnostic procedures:
39 (29.3%) cases via bronchoscopy with or without
endobronchial ultrasound; 32 (24.1%) via CT-guided
biopsy of a lung lesion; 44 (33.1%) through surgical
biopsy; nine (6.8%) through cytological analysis of
sputum, pleural fluid, ascites, or pericardial effusion;
seven (5.3%) by ultrasound-guided fine needle biopsy
of cervical lymph nodes or subcutaneous nodules;
one (0.8%) via endoscopic ultrasound-guided biopsy
of an adrenal lesion; and one (0.8%) through liquid
biopsy detecting EGFR mutations as the patient
declined tissue biopsy (online supplementary Fig 2).
Of the 133 patients, 17 (12.8%) underwent invasive
investigations at metastatic sites.
Lung cancer stages
Among the 133 lung cancer cases, 62 (46.6%) were
stage I, 10 (7.5%) were stage II, 16 (12.0%) were
stage III, and 45 (33.8%) were stage IV. No cases of
adenocarcinoma in situ arising from GGOs were
identified among the 291 patients who underwent
invasive investigations; however, five cases of
minimally invasive adenocarcinoma (pT1mi,
pathological stage IA1) were detected. The proportion
of stage I lung cancer in the lung nodule surveillance
programme (46.6%) was significantly higher than
that reported by the Hong Kong Cancer Registry
(HKCR) in 2021 (17.4%, P<0.001).1 Conversely, the
proportion of stage IV cases was significantly lower
in the surveillance programme (33.8%, 45 of 133)
compared with the HKCR data (56.9%, P<0.001)
[Fig 2a]. Overall, 54.1% (72 of 133) of cases in the
surveillance programme were diagnosed at an early
stage (stage I-II), compared with 22.4% in the HKCR
data (P<0.001) [Fig 2b].
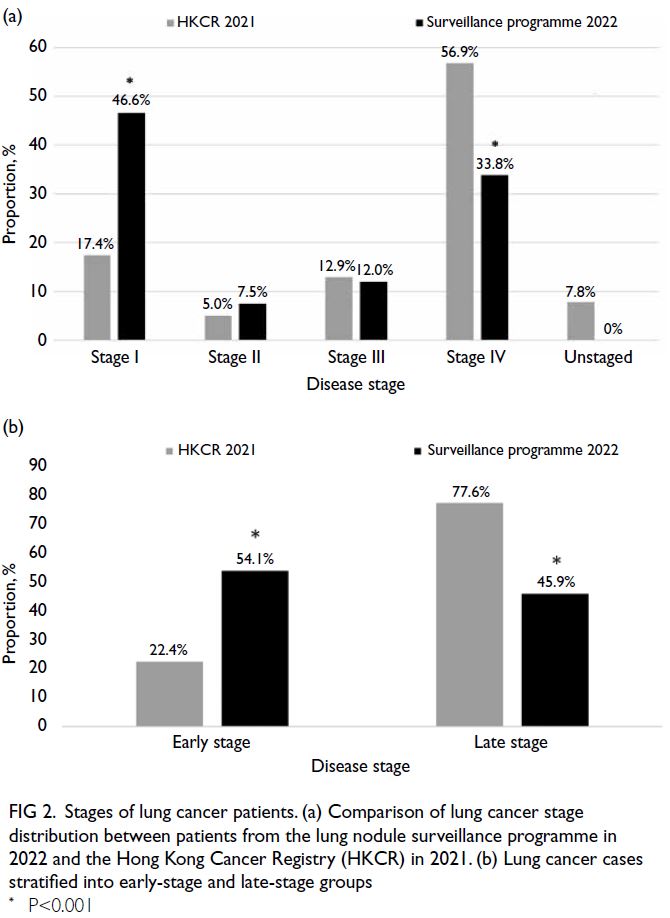
Figure 2. Stages of lung cancer patients. (a) Comparison of lung cancer stage distribution between patients from the lung nodule surveillance programme in 2022 and the Hong Kong Cancer Registry (HKCR) in 2021. (b) Lung cancer cases stratified into early-stage and late-stage groups
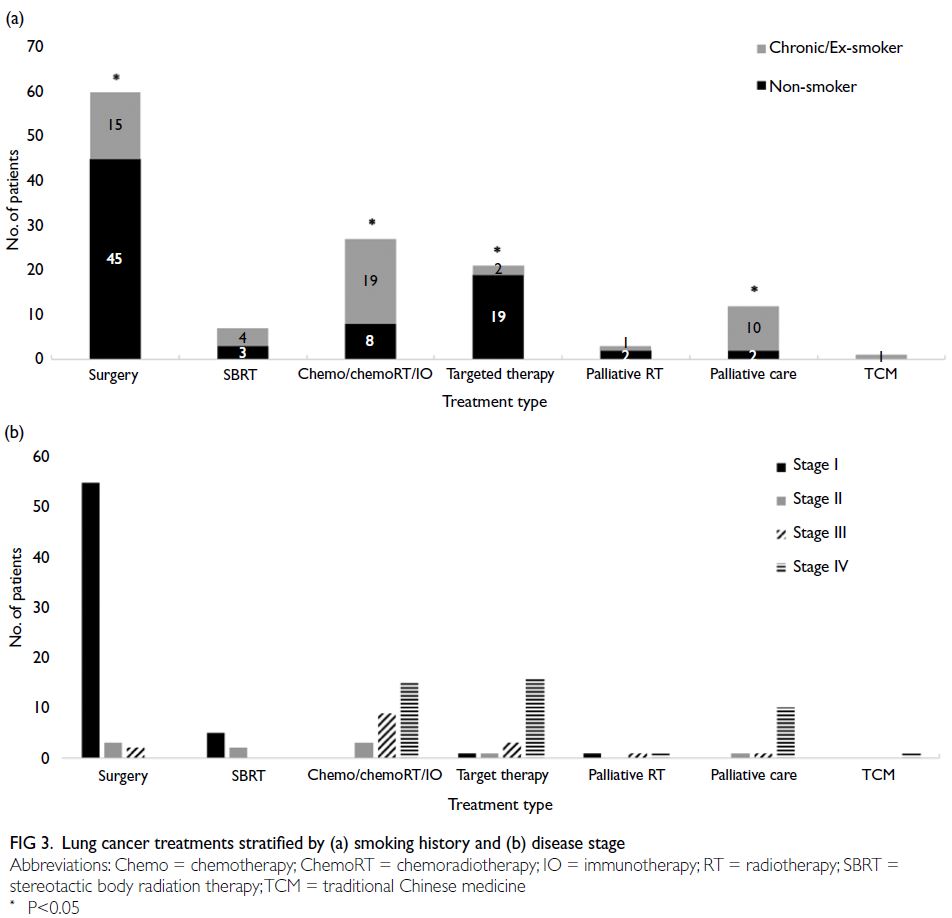
Lung cancer treatments
Of the 133 lung cancer patients, 60 (45.1%)
underwent surgical resection; seven (5.3%) received
stereotactic body radiation therapy; 27 (20.3%) were treated with systemic chemotherapy with or without
radiotherapy, combined chemoimmunotherapy
(chemoIO), or IO alone; and 21 (15.8%) received
targeted therapy. Three patients (2.3%) received
palliative radiotherapy as their first-line treatment.
The remaining 12 (9.0%) patients opted for best
supportive care, one (0.8%) patient pursued
traditional Chinese medicine, and two moved out of
Hong Kong. Two cases who left Hong Kong were lost
to follow-up after diagnosis.
Compared with non-smokers, a lower
proportion of patients with a history of smoking
underwent surgical resection (45 of 79 [57.0%]
non-smokers vs 15 of 52 [28.8%] current/ex-smokers,
P=0.0014) and targeted therapy (19 of 79
[24.1%] vs 2 of 52 [3.8%], P=0.0019). In contrast, a
higher proportion of current/ex-smokers received
chemotherapy, chemoradiotherapy, chemoIO, or IO
alone (8 of 79 [10.1%] vs 19 of 52 [36.5%], P<0.001)
and best supportive care (2 of 79 [2.5%] vs 10 of 52 [19.2%], P=0.0014) [Fig 3a]. Patients diagnosed
with stage I lung cancer primarily received surgical
resection or curative RT, whereas those diagnosed at
stage III or IV were more likely to receive systemic
therapy or best supportive care (Fig 3b).
Lung cancer patient survival
As of the survival analysis conducted in April 2024,
the median survival for all patients had not been
reached. Among patients with late-stage lung cancer,
the median PFS was 282 days (95% confidence
interval [95% CI]=52-512), and the median OS
was 438 days (95% CI=137-739). Both the median
disease-free survival and OS for patients with early-stage
lung cancer had not been reached at the time
of analysis (Fig 4).
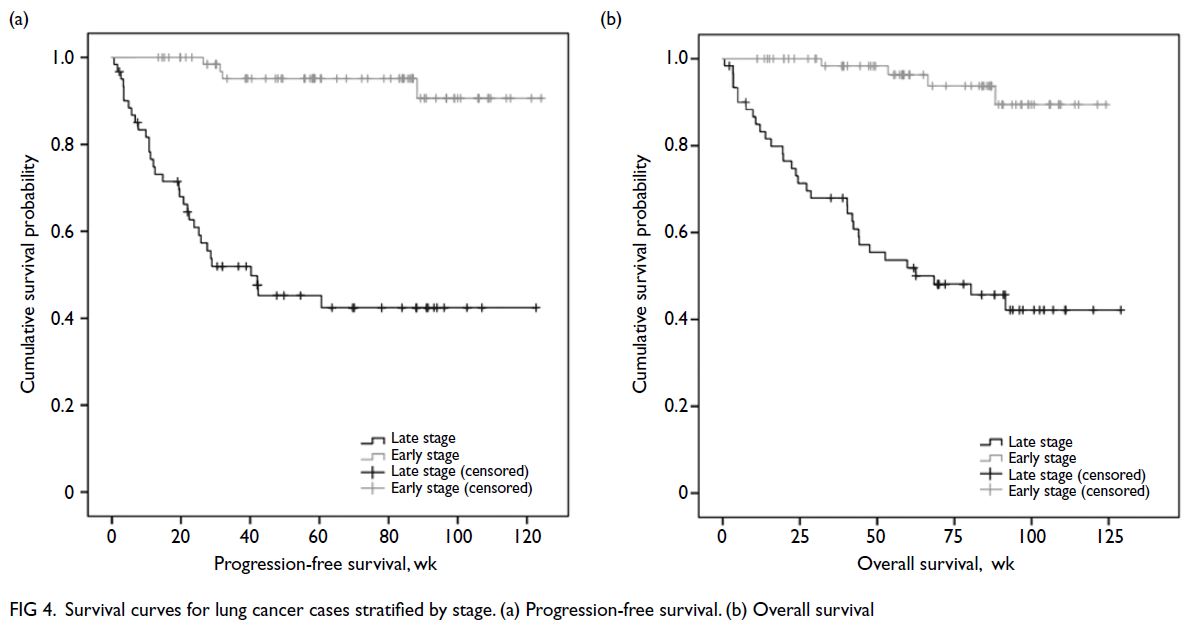
Figure 4. Survival curves for lung cancer cases stratified by stage. (a) Progression-free survival. (b) Overall survival
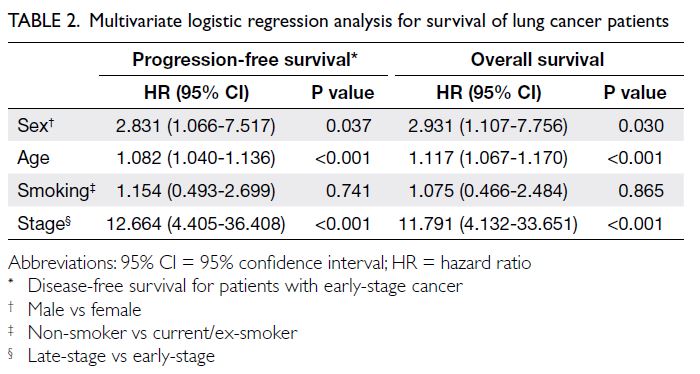
Univariate Cox regression analysis revealed that female sex (P<0.02), age (P<0.001), smoking
history (P<0.02), lung lesion size (P≤0.001), disease
stage (P<0.001), and surgical resection (P<0.001)
were significant predictors of both PFS and OS.
Multivariate Cox regression analysis showed
that male sex, age, and late-stage disease were
independently associated with survival. For male sex,
the hazard ratios were 2.831 (95% CI=1.066-7.517)
for PFS and 2.931 (1.107-7.756) for OS (P=0.037
and 0.030, respectively). For age, the hazard ratios
were 1.082 (1.040-1.136) for PFS and 1.117 (1.067-1.170) for OS, and for late-stage disease, the hazard
ratios were 12.664 (4.405-36.408) and 11.791 (4.132-33.651) for PFS and OS, respectively (all P<0.001)
[Table 2]. There were no statistically significant
differences in survival rates based on nodule
characteristics or locations.
Discussion
In this single-centre study, the implementation of
a lung nodule surveillance programme resulted in
54.1% of lung cancer patients being diagnosed at an
early, potentially curable stage, which may contribute
to improved survival outcomes. Compared with
HKCR 2021 data, this reflects a 31.7% increase
in the proportion of early-stage diagnoses (from
22.4% to 54.1%) and a potential 23.1% reduction in
the proportion of patients diagnosed with stage IV
disease (from 56.9% to 33.8%).
The prevalence of lung nodules detected in
lung cancer screening trials varies considerably, with
reported rates ranging from 6.8% to 50.9%.7 14 15 16 17 18 19 The
National Lung Screening Trial showed that 96% of
nodules detected in high-risk smokers were non-malignant.2 The Taiwan Lung Cancer Screening in
Never-Smoker Trial, a multicentre cohort of 12 011
never-smokers, revealed a lung nodule prevalence
of 17.4% and a cancer prevalence of 2.6% at baseline
screening.20 An ongoing clinical trial in Hong Kong
is investigating lung cancer screening in individuals
with a smoking history or a family history of
lung cancer.21 Although low-dose CT screening
is a proven modality that significantly reduces
lung cancer mortality in some countries, current
guidelines recommend screening only for a limited
high-risk population. Moreover, the detection of
incidental lung nodules has increased with the
widespread use of CT scans.22 In our study, such
nodules accounted for 57.5% of all cases. Increased referrals for incidental nodules likely contributed to
longer waiting times for follow-up appointments.
Previous studies have reported delayed or failed
tracking of incidental pulmonary nodules in 40% to
70% of patients,8 23 and one study reported a 27% loss
to follow-up.19 A dedicated lung nodule surveillance
programme can complement lung cancer screening
by facilitating early detection in individuals who do
not meet current screening criteria but present with
incidental pulmonary nodules. In our study, overdue
scans (9.0%) and missed follow-up appointments
(23.3%) were promptly identified and rescheduled by
programme coordinators, ensuring close monitoring
and timely intervention to optimise patient
outcomes.
Lung nodule surveillance programmes have
shown success across various settings. For example,
the lung nodule registry at National Jewish Health
established a database of patients with identified
nodules, resulting in improved follow-up imaging
and a stage shift towards earlier lung cancer
diagnosis.24 25 Similarly, a comprehensive programme
in Tennessee in the US reported an increase in
the proportion of stage I or II cancer diagnoses
from 23% to 38% following the implementation of
electronic and manual chart reviews.10 The lung
nodule surveillance programme in this study not
only actively tracked reports but also provided
clinical assessments, estimated patient cancer risk,
and optimised guideline adherence. Our findings
echo those of previous studies: stage I and II lung
cancer was detected in 54.1% of cases, compared
with 22.4% reported in the 2021 HKCR report.
Smoking remains a significant risk factor for
lung cancer in both men and women.26 Our study
showed that smokers presented with larger nodules,
more advanced disease stages, and a lower likelihood
of undergoing surgical resection compared with
non-smokers. Unlike Western populations where
10% to 20% of lung cancer patients are non-smokers,27 approximately 38% of lung cancer cases
in Asia occur in non-smokers.2 18 28 29 30 31 32 33 34 35 Our findings
reflect this regional trend, with 60.9% of confirmed
lung cancer patients being non-smokers; among
them, 38.3% were diagnosed at a late stage. Non-smokers
in our cohort were more likely to have
GGOs, adenocarcinoma, EGFR mutations, and
early-stage lung cancer at diagnosis—findings
consistent with previous studies.23 35 36 37 These results
suggest that lung cancer control efforts in Hong
Kong should target both smokers and non-smokers
with relevant risk factors.5 Beyond smoking status,
emerging research highlighted sex-based differences
in lung cancer biology, including hormonal factors,
molecular changes, genetic predispositions, the
presence of human papillomavirus, nontuberculous
mycobacteria, and prior radiation therapy for breast
cancer.35
Previous studies have shown that improved
survival is associated with stage shifts.38 39 For
example, Yang et al5 reported an increase in stage
I disease from 15.9% to 58.8% and a decrease in
stage IV disease from 60.3% to 25.7% between
2006 and 2019, during which the 5-year survival
rate for lung cancer in Taiwan rose from 22.1% to
54.9%. In the present study, although the median
PFS and OS had not yet been reached at the time
of analysis; patients with early-stage lung cancer had
significantly better survival outcomes. Multivariate
analysis identified sex, stage, and age as independent
predictors of survival, while no significant survival
differences were observed based on nodule density
or location. These findings suggest that although the physical attributes of lung nodules are important
for diagnosis, survival outcomes are more strongly
influenced by factors such as stage at diagnosis,
patient demographics, and treatment strategies.
Limitation
This study has several limitations. First, the follow-up
period was relatively short at just over 15 months,
and the median PFS and OS were not reached for
early-stage patients. Second, the observational
design of this single-centre study limits the ability
to establish a causal relationship between the lung
nodule surveillance programme and the observed
stage shift. Although patients with clinically
metastatic lung cancer were not excluded, the study
cohort may not fully represent the broader lung
cancer population in Hong Kong. Nevertheless,
the findings provide indirect evidence that a lung
nodule surveillance programme may facilitate early-stage
detection. To confirm the programme’s impact
on stage shift and survival outcomes, randomised
controlled trials or case-control studies comparing
enrolled patients with non-enrolled but eligible
patients are warranted. Furthermore, local studies
evaluating the cost-effectiveness of such programmes
would provide further evidence to optimise care for
patients with lung nodules.
Conclusion
This lung nodule surveillance programme improved
follow-up compliance and facilitated timely,
appropriate investigations for high-risk patients.
Consequently, more than half of lung cancer cases
were diagnosed at an early stage, enabling provision
of curative treatments and potentially contributing
to improved survival outcomes.
Author contributions
Concept or design: DCL Lam, LYW Shong.
Acquisition of data: WC Chong, LYW Shong, PI Cheang, WC Choy.
Analysis or interpretation of data: LYW Shong, FKP Chan, WC Kwok, WC Chong, MSM Ip, DCL Lam.
Drafting of the manuscript: DCL Lam and LYW Shong.
Critical revision of the manuscript for important intellectual content: DCL Lam, LYW Shong, WC Kwok.
Acquisition of data: WC Chong, LYW Shong, PI Cheang, WC Choy.
Analysis or interpretation of data: LYW Shong, FKP Chan, WC Kwok, WC Chong, MSM Ip, DCL Lam.
Drafting of the manuscript: DCL Lam and LYW Shong.
Critical revision of the manuscript for important intellectual content: DCL Lam, LYW Shong, WC Kwok.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
As an editor of the journal, WC Kwok was not involved in
the peer review process. Other authors have disclosed no
conflicts of interest.
Acknowledgement
The authors thank all patients for their participation.
Declaration
Part of the research data was presented at the Hospital
Authority Convention 2024, 16-17 May 2024, Hong Kong.
Funding/support
The research described in this report was supported in part
by the Lee and the Ho Families Respiratory Research Fund.
Ethics approval
This study was approved by the Institutional Review Board
of The University of Hong Kong/Hospital Authority Hong
Kong West Cluster, Hong Kong (Ref No.: UW23-101) and was
conducted in full compliance with the ICH E6 guideline for
Good Clinical Practice and the principles of the Declaration
of Helsinki. The requirement for patient consent was waived
by the ethics board as the study involved minimal risk and
used data collected during routine clinical care without
altering patient management.
Supplementary material
The supplementary material was provided by the authors and
some information may not have been peer reviewed. Accepted
supplementary material will be published as submitted by the
authors, without any editing or formatting. Any opinions
or recommendations discussed are solely those of the
author(s) and are not endorsed by the Hong Kong Academy
of Medicine and the Hong Kong Medical Association.
The Hong Kong Academy of Medicine and the Hong Kong
Medical Association disclaim all liability and responsibility
arising from any reliance placed on the content.
References
1. Hospital Authority. Hong Kong Cancer Registry 2021.
Available from: https://www3.ha.org.hk/cancereg/. Accessed 19 Apr 2024.
2. Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer
mortality with low-dose computed tomographic
screening. N Engl J Med 2011;365:395-409. Crossref
3. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced
lung-cancer mortality with volume CT screening in a
randomized trial. N Engl J Med 2020;382:503-13. Crossref
4. Becker N, Motsch E, Trotter A, et al. Lung cancer
mortality reduction by LDCT screening—results
from the randomized German LUSI trial. Int J Cancer
2020;146:1503-13. Crossref
5. Yang CY, Lin YT, Lin LJ, et al. Stage shift improves lung
cancer survival: real-world evidence. J Thorac Oncol
2023;18:47-56. Crossref
6. Centre for Health Protection, Department of Health, Hong
Kong SAR Government. Cancer Expert Working Group
on Cancer Prevention and Screening. Recommendations
on Prevention and Screening for Lung Cancer For Health
Professionals. Jun 2023. Available from: https://www.chp.gov.hk/files/pdf/lung_cancer_professional_hp.pdf. Accessed 31 Aug 2024.
7. Gould MK, Tang T, Liu IL, et al. Recent trends in the
identification of incidental pulmonary nodules. Am J
Respir Crit Care Med 2015;192:1208-14. Crossref
8. Shelver J, Wendt CH, McClure M, et al. Effect of an
automated tracking registry on the rate of tracking failure in incidental pulmonary nodules. J Am Coll Radiol
2017;14:773-7. Crossref
9. Wiener RS, Gould MK, Slatore CG, Fincke BG, Schwartz LM,
Woloshin S. Resource use and guideline concordance in
evaluation of pulmonary nodules for cancer: too much and
too little care. JAMA Intern Med 2014;174:871-80. Crossref
10. LeMense GP, Waller EA, Campbell C, Bowen T.
Development and outcomes of a comprehensive
multidisciplinary incidental lung nodule and lung cancer
screening program. BMC Pulm Med 2020;20:115. Crossref
11. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for
management of incidental pulmonary nodules detected on
CT images: from the Fleischner Society 2017. Radiology
2017;284:228-43. Crossref
12. Bai C, Choi CM, Chu CM, et al. Evaluation of pulmonary
nodules: clinical practice consensus guidelines for Asia.
Chest 2016;150:877-93. Crossref
13. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The
eighth edition lung cancer stage classification. Chest
2017;151:193-203. Crossref
14. Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of
CT screening for lung cancer: a systematic review. JAMA
2012;307:2418-29. Crossref
15. Field JK, Duffy SW, Baldwin DR, et al. UK Lung Cancer RCT
Pilot Screening Trial: baseline findings from the screening
arm provide evidence for the potential implementation of
lung cancer screening. Thorax 2016;71:161-70. Crossref
16. Pedersen JH, Ashraf H, Dirksen A, et al. The Danish
randomized lung cancer CT screening trial—overall
design and results of the prevalence round. J Thorac Oncol
2009;4:608-14. Crossref
17. Horeweg N, Scholten ET, de Jong PA, et al. Detection of
lung cancer through low-dose CT screening (NELSON):
a prespecified analysis of screening test performance and
interval cancers. Lancet Oncol 2014;15:1342-50. Crossref
18. Krist AH, Davidson KW, Mangione CM, et al. Screening
for lung cancer: US Preventive Services Task Force
recommendation statement. JAMA 2021;325:962-70. Crossref
19. Weinstock TG, Tewari A, Patel H, et al. No stone unturned:
Nodule Net, an intervention to reduce loss to follow-up of
lung nodules. Respir Med 2019;157:49-51. Crossref
20. Chang GC, Chiu CH, Yu CJ, et al. Low-dose CT screening
among never-smokers with or without a family history of
lung cancer in Taiwan: a prospective cohort study. Lancet
Respir Med 2024;12:141-52. Crossref
21. ClinicalTrials.gov. Screening for lung cancer in subjects with
family history of lung cancer (Identifier NCT05762731).
2023. Available from: https://www.clinicaltrials.gov/study/NCT05762731 . Accessed 19 Apr 2024.
22. Schmid-Bindert G, Vogel-Claussen J, Gütz S, et al.
Incidental pulmonary nodules—what do we know in 2022.
Respiration 2022;101:1024-34. Crossref
23. Digby GC, Habert J, Sahota J, Zhu L, Manos D. Incidental
pulmonary nodule management in Canada: exploring
current state through a narrative literature review and
expert interviews. J Thorac Dis 2024;16:1537-51. Crossref
24. Carr LL, Dyer DS, Zelarney PT, Kern EO. Improvement in
stage of lung cancer diagnosis with incidental pulmonary
nodules followed with a patient tracking system and
computerized registry. JTO Clin Res Rep 2022;3:100297. Crossref
25. Dyer DS, Zelarney PT, Carr LL, Kern EO. Improvement
in follow-up imaging with a patient tracking system and
computerized registry for lung nodule management. J Am
Coll Radiol 2021;18:937-46. Crossref
26. O’Keeffe LM, Taylor G, Huxley RR, Mitchell P, Woodward M,
Peters SA. Smoking as a risk factor for lung cancer in
women and men: a systematic review and meta-analysis.
BMJ Open 2018;8:e021611. Crossref
27. McCarthy WJ, Meza R, Jeon J, Moolgavkar SH. Chapter
6: Lung cancer in never smokers: epidemiology and risk
prediction models. Risk Anal 2012;32 Suppl 1(Suppl 1):S69-84. Crossref
28. Church TR, Black WC, Aberle DR, et al. Results of initial
low-dose computed tomographic screening for lung
cancer. N Engl J Med 2013;368:1980-91. Crossref
29. Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting
of low-dose CT screening according to the risk of lung-cancer
death. N Engl J Med 2013;369:245-54. Crossref
30. Aberle DR, DeMello S, Berg CD, et al. Results of the two
incidence screenings in the National Lung Screening Trial.
N Engl J Med 2013;369:920-31. Crossref
31. Patz EF Jr, Pinsky P, Gatsonis C, et al. Overdiagnosis in
low-dose computed tomography screening for lung cancer.
JAMA Intern Med 2014;174:269-74. Crossref
32. Hong Kong Cancer Registry, Hospital Authority. Cancer
Facts. 2020. Available from: https://www3.ha.org.hk/cancereg/facts.html. Accessed 19 Apr 2024.
33. Cho J, Choi SM, Lee J, et al. Proportion and clinical features of never-smokers with non–small cell lung cancer. Chin J Cancer 2017;36:20.Crossref
34. Chinese Expert Group on Early Diagnosis and Treatment
of Lung Cancer, China Lung Oncology Group. China
National Lung Cancer Screening Guideline with Lowdose
Computed Tomography (2023 Version) [in Chinese].
Zhongguo Fei Ai Za Zhi 2023;26:1-9. Crossref
35. MacRosty CR, Rivera MP. Lung cancer in women: a
modern epidemic. Clin Chest Med 2020;41:53-65. Crossref
36. Horeweg N, van Rosmalen J, Heuvelmans MA, et al.
Lung cancer probability in patients with CT-detected
pulmonary nodules: a prespecified analysis of data from
the NELSON trial of low-dose CT screening. Lancet Oncol
2014;15:1332-41. Crossref
37. Tolwin Y, Gillis R, Peled N. Gender and lung cancer–SEER-based
analysis. Ann Epidemiol 2020;46:14-9. Crossref
38. Flores R, Patel P, Alpert N, Pyenson B, Taioli E. Association
of stage shift and population mortality among patients
with non–small cell lung cancer. JAMA Netw Open
2021;4:e2137508. Crossref
39. Potter AL, Rosenstein AL, Kiang MV, et al. Association of
computed tomography screening with lung cancer stage
shift and survival in the United States: quasi-experimental
study. BMJ 2022;376:e069008. Crossref