Hong Kong Med J 2025;31:Epub 12 Jun 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Living donor renal transplantation in a patient with human immunodeficiency virus: a case report
TL Leung, MB ChB, FHKAM (Medicine)1; Jacky MC Chan, FHKAM (Medicine), FRCP2; Ivy LY Wong, FHKAM (Medicine), FHKCP1; Clara KY Poon, FHKAM (Medicine), FRCP1; William Lee, FHKAM (Medicine), FHKCP1; KF Yim, FHKAM (Medicine), FHKCP1; Owen TY Tsang, FHKAM (Medicine), FRCP2; Samuel KS Fung, FHKAM (Medicine), FRCP1; A Cheuk, FHKAM (Medicine), FRCP1; HL Tang, FHKAM (Medicine), FRCP1
1 Renal Unit, Department of Medicine and Geriatrics, Princess Margaret Hospital, Hong Kong SAR, China
2 Infectious Disease Unit, Department of Medicine and Geriatrics, Princess Margaret Hospital, Hong Kong SAR, China
Corresponding author: Dr TL Leung (ltl125@ha.org.hk)
Case presentation
The patient was diagnosed in 1989, at age 27 years,
with human immunodeficiency virus-1 (HIV-1)
infection. He later presented with hypertension and
left loin discomfort. Workup revealed proteinuria of
0.51 g over 24 hours and impaired renal function,
with serum creatinine of 230 μmol/L (normal range,
59-104 μmol/L). An ultrasound scan showed bilateral
shrunken kidneys with loss of corticomedullary
differentiation. Renal biopsy was not performed
in view of the bilateral shrunken kidneys. His
renal function progressively deteriorated, and he
commenced automated peritoneal dialysis in June
2017 at age 55 years. His elder brother volunteered
to donate a kidney. The patient’s HIV infection was
well controlled with lamivudine, abacavir, lopinavir,
and ritonavir. Lopinavir/ritonavir was switched to
raltegravir in view of a potential drug-drug interaction
between ritonavir and calcineurin inhibitors. Pre-transplantation
CD4+ count was 717 cells/μL and
HIV viral load was undetectable. Human leukocyte
antigen matching revealed one mismatch between
donor and recipient. Both donor and recipient tested
cytomegalovirus antibody positive. The recipient
was started on cyclosporine, mycophenolate mofetil,
and prednisolone for immunosuppression according
to our centre’s protocol.
Living donor renal transplantation was
successfully performed in September 2018
when the patient was 56 years old. Postoperative
ultrasound of the graft kidney and radioisotope
scan showed good graft perfusion and function.
Valganciclovir and pentamidine inhalation were
given as prophylaxis against cytomegalovirus and
Pneumocystis jirovecii, respectively, in view of his
underlying glucose-6-phosphatase deficiency. The
lowest creatinine level since hospital discharge was
112 μmol/L. At 6 months post-transplantation,
he was found to have a low level of donor-specific
anti-DQ7 antibody (DSA), which persisted at repeat
testing 9 months post-transplantation. Cyclosporine was therefore switched to tacrolimus to optimise
immunosuppression. The tacrolimus trough level has
been maintained at 5 to 8 μg/L since commencement
(Fig a). Despite the presence of DSA, the patient has
enjoyed stable renal function with no proteinuria;
thus, renal biopsy was not performed (Fig b). In his
latest follow-up, at 51 months post-transplantation,
his renal function remained stable with a creatinine
level of 141 μmol/L. He also has excellent HIV control
with abacavir, dolutegravir, and lamivudine. CD4+
cell count has been maintained in the range of 600 to
800 cells/μL since transplantation (Fig c). He did not
experience any infections after transplantation.
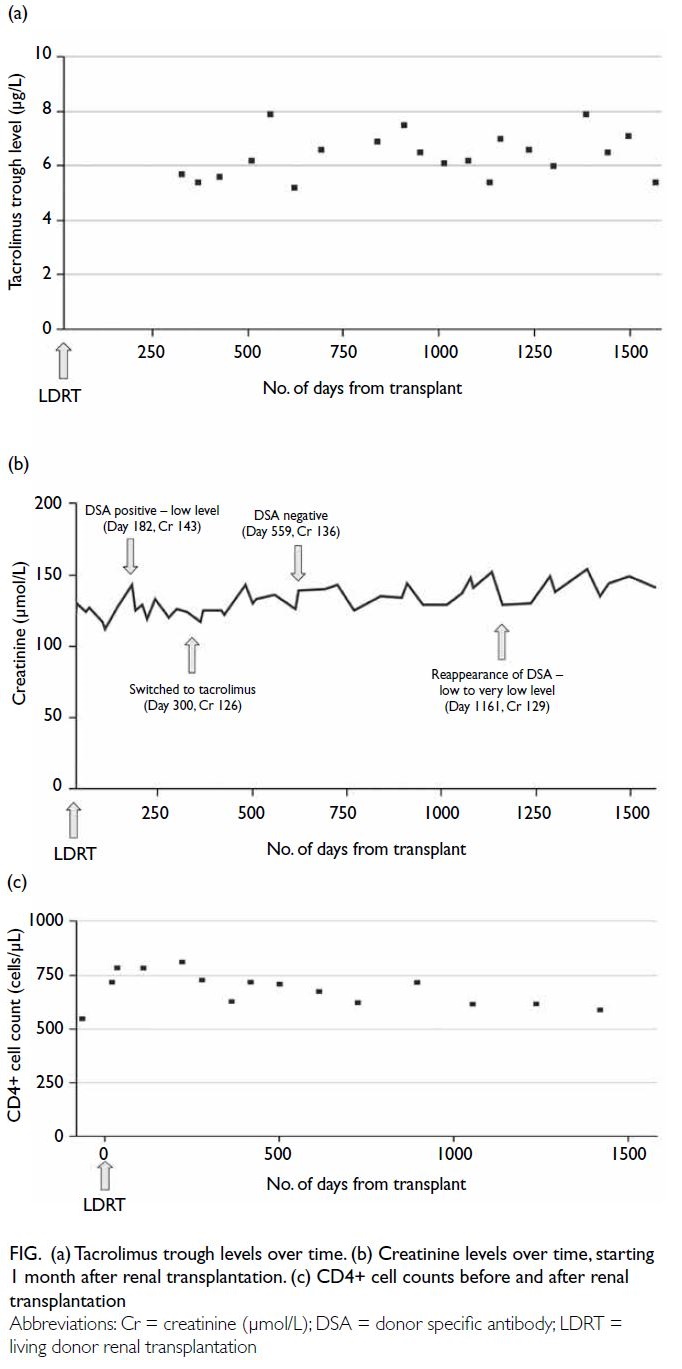
Figure. (a) Tacrolimus trough levels over time. (b) Creatinine levels over time, starting 1 month after renal transplantation. (c) CD4+ cell counts before and after renal transplantation
Discussion
To the best of our knowledge, this is the first reported
case of living donor renal transplantation in a patient
with HIV in Hong Kong. The global prevalence of
HIV is increasing, and its association with chronic
kidney disease is notable. In a local cohort, 16.8%
of Chinese HIV-infected patients developed
chronic kidney disease.1 With the advancement
of highly active antiretroviral therapy (HAART),
life expectancy among people living with HIV
(PLHIV) approaches that of the general population,
leading to more cases of end-stage renal failure
requiring management. The combination of intense
immunosuppression and intrinsic immunodeficiency
can expose HIV-infected transplant recipients
to life-threatening opportunistic infections. It is
crucial to achieve excellent HIV control before
proceeding with transplantation. The American
Society of Transplantation recommends that PLHIV
achieve a CD4+ cell count of more than 200 cells/μL during the 3 months prior to transplantation
and an undetectable HIV viral load while receiving
HAART.2 Our patient met these criteria prior to
transplantation.
Kidney transplantation in PLHIV has been
explored in many Western countries over the past decades. In a systematic review, the 1- and 3-year
patient survival rates after transplantation were
reported at 97% and 94%, respectively, with graft
survival at 91% and 81%.3 In another cohort study that compared 510 HIV-infected kidney transplant
recipients with HIV-negative controls, comparable
5- and 10-year survival rates were reported; 5-year
patient and graft survival were 88.7% and 75%,
respectively. Co-infection of HIV and hepatitis C
virus was an important prognostic marker for poor
graft and patient survival.4 These excellent survival
data encouraged us to perform the first kidney
transplant in an HIV-infected patient. Despite these
promising results, the rejection rate in HIV-infected
recipients is significantly higher, up to 2- to 3-fold,
potentially due to drug-drug interactions between
HAART and immunosuppressants or immune
dysregulation.5 6 The pathophysiology behind this
increased rejection rate is unclear, but strategies
such as induction therapy with anti-interleukin-2
receptor antibody or antithymocyte globulin and
optimised immunosuppressive therapy are utilised to
mitigate risks. Nonetheless, the use of antithymocyte
globulin is controversial due to its association with
marked CD4+ cell suppression (ie, <200 cells/μL),
prolonged recovery, and subsequent infection risk.7
Therefore, antithymocyte globulin induction should
be reserved for HIV-infected recipients with very
high immunological risk.
The optimal immunosuppression regimen for
HIV-infected recipients is yet to be determined.
Tacrolimus is favoured over cyclosporine for
reducing acute rejection risks.8 Observational studies
and the landmark ELITE-Symphony trial suggest
lower rejection rates with tacrolimus, up to 2-fold.6 8 Our centre initially chose cyclosporine due
to the patient’s low immunological risk profile with
only one human leukocyte antigen mismatch but
switched to tacrolimus after the development of DSA
6 months post-transplantation. This case highlights
the challenges of balancing overimmunosuppression
and rejection risks in HIV-infected recipients.
Ongoing evidence supports the use of tacrolimus as
the first-line immunosuppressive agent, irrespective
of immunological risk, with future randomised
controlled trials needed to establish the best regimen.
Managing drug-drug interactions in HIV-infected
transplant recipients is complex. Non-nucleoside
reverse transcriptase inhibitors induce
cytochrome P450 enzymes, while protease inhibitors
significantly inhibit these enzymes, notably raising
calcineurin inhibitor levels in the plasma. Ritonavir,
a strong CYP3A4 inhibitor commonly used in
HAART, requires a substantial increase in tacrolimus
dosage up to 70-fold upon its discontinuation to
maintain effective immunosuppression.9 To avoid
drug-drug interactions, our patient was switched to
an integrase strand transfer inhibitor-based HAART
regimen prior to transplantation. Nonetheless, there
was a risk of serum creatinine elevation. Dolutegravir
has been shown to inhibit organic cation transporter
2, which inhibits active creatinine secretion into renal tubules, leading to a slight elevation in serum
creatinine level without affecting the glomerular
filtration rate. After administering dolutegravir,
serum creatinine clearance in healthy subjects has
been reported to decrease by 10% to 14%.10
In conclusion, renal transplantation in PLHIV
can offer improved quality of life and survival
compared with continued dialysis, provided there
is excellent HIV control and careful management
of immunosuppression and drug-drug interactions.
Challenges remain in preventing and treating acute
rejection to improve long-term graft survival. We
observed an early appearance of DSA 6 months post-transplantation
in our patient. Although the DSA was
transiently suppressed after switching to tacrolimus,
it reappeared later. The appearance of DSA and its
potential long-term impact on graft survival require
further investigation. Our experience, alongside data
from Western cohorts, supports expanding renal
transplantation among HIV-infected patients, with a
focus on tailored immunosuppressive strategies and
management of complications.
Author contributions
All authors contributed to the concept or design of the study,
acquisition of the data, analysis or interpretation of the
data, drafting of the manuscript, and critical revision of the
manuscript for important intellectual content. All authors
had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Declaration
This case has been presented during oral presentation at
the 18th Congress of The Asian Society of Transplantation
(CAST) in Hong Kong SAR, China, 25-28 July 2023.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki and provided informed consent for all procedures.
References
1. Cheung CY, Wong KM, Lee MP et al. Prevalence of chronic
kidney disease in Chinese HIV-infected patients. Nephrol
Dial Transplant 2007;22:3186-90. Crossref
2. Blumberg EA, Rogers CC; American Society of
Transplantation Infectious Diseases Community of
Practice. Solid organ transplantation in the HIV-infected
patients: Guidelines from the American Society of
Transplantation Infectious Diseases Community of
Practice. Cli Transplant 2019;33:e13499. Crossref
3. Zheng X, Gong L, Xue W, et al. Kidney transplant outcomes
in HIV-positive patients: a systematic review and meta-analysis.
AIDS Res Ther 2019;16:37. Crossref
4. Locke JE, Mehta S, Reed RD, et al. A national study
of outcomes among HIV-infected kidney transplant
recipients. J Am Soc Nephrol 2015;26:2222-9. Crossref
5. Stock PG, Barin B, Murphy B, et al. Outcomes of kidney
transplantation in HIV-infected recipients. N Engl J Med
2010;363:2004-14. Crossref
6. Gathogo E, Harber M, Bhagani S, et al. Impact of tacrolimus
compared with cyclosporin on the incidence of acute
allograft rejection in Human Immunodeficiency virus-positive
kidney transplant recipients. Transplantation
2016;100:871-8. Crossref
7. Carter JT, Melcher ML, Carlson LL, Roland ME, Stock PG.
Thymoglobulin-associated Cd4+ T-cell depletion and
infection risk in HIV-infected renal transplant recipients.
Am J Transplant 2006;6:753-60. Crossref
8. Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced
exposure to calcineurin inhibitors in renal transplantation.
N Engl J Med 2007;357:2562-75. Crossref
9. Jimenez HR, Natali KM, Zahran AA. Drug interaction after
ritonavir discontinuation: considerations for antiretroviral
therapy changes in renal transplant recipients. Int J STD
AIDS 2019;30:710-4. Crossref
10. Koteff J, Borland J, Chen S, et al. A phase 1 study to evaluate
the effect of dolutegravir on renal function via measurement
of iohexol and para-aminohippurate clearance in healthy
subjects. Br J Cli Pharmacol 2013;75:990-6. Crossref

