Hong Kong Med J 2025;31:Epub 5 Dec 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Specific indicators of unsuitability for
transarterial chemoembolisation in patients with
intermediate-stage hepatocellular carcinoma
according to thresholds of tumour burden and
liver function as judged by survival benefit over
sorafenib
LM Chen, PhD1,2,3; Simon CH Yu, MB, BS, MD1; Leung Li, MB, ChB, MD4; Edwin P Hui, MB, ChB, MD4; Winnie Yeo, MB, BS, MD4,5; Stephen L Chan, MB, BS, MD4,5
1 Department of Imaging and Interventional Radiology, Faculty of
Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
2 Department of Medical Ultrasonics, The Sixth Affiliated Hospital, Sun
Yat-sen University, Guangzhou, China
3 Biomedical Innovation Center, The Sixth Affiliated Hospital, Sun Yat-sen
University, Guangzhou, China
4 Department of Clinical Oncology, Prince of Wales Hospital, The Chinese
University of Hong Kong, Hong Kong SAR, China
5 State Key Laboratory of Translational Oncology, China
Corresponding author: Dr Simon CH Yu (simonyu@cuhk.edu.hk)
Abstract
Introduction: This study aimed to define specific
indicators of unsuitability for transarterial
chemoembolisation (TACE) in patients with
intermediate-stage hepatocellular carcinoma (HCC)
in Hong Kong using thresholds of tumour burden
and liver function, as judged by survival benefit over
sorafenib.
Methods: Patients with treatment-naïve and
unresectable HCC who received TACE or sorafenib
from 2005 to 2019 and met the eligibility criteria
were enrolled. Overall survival (OS) was compared
between the TACE and sorafenib groups using
the log-rank test and hazard ratios (HRs) in all
subgroups classified according to baseline modified
albumin–bilirubin (mALBI) grade and tumour
burden, including the up-to-7, up-to-11, and N3-S5-S10 criteria.
Results: Overall survival was significantly longer in
TACE subgroups than in sorafenib subgroups when
stratified by mALBI grade and either the up-to-7 or
the up-to-11 criteria (all P<0.05). When applying
the N3-S5-S10 criteria, OS did not significantly
differ between the TACE and sorafenib groups in
subgroups with mALBI grade 2b and tumours with
number >3 and size >5 cm but ≤10 cm, or tumours
with number >3 and size >10 cm (HR=0.550 and
0.965, respectively; both P>0.05). Sensitivity analysis
showed non-significant survival benefits in two
additional subgroups: those with mALBI grade 2b and tumours with number ≤3 and size >10 cm, and
those with mALBI grade 1 or 2a and tumours with
number >3 and size >10 cm (HR=0.474 and 0.418,
respectively; both P>0.05).
Conclusion: More precise criteria for TACE
unsuitability are required. The combination of
mALBI grade and the N3-S5-S10 criteria may better
identify patients with intermediate-stage HCC who
are unlikely to benefit from TACE. Validation in a
larger cohort is warranted.
New knowledge added by this study
- Patients regarded as unsuitable for transarterial chemoembolisation (TACE) under existing criteria may achieve better survival outcomes with TACE than those with systemic therapy.
- To determine true TACE unsuitability, more precise criteria based on clinical evidence demonstrating improved survival with alternative treatments are required. Modified albumin–bilirubin (mALBI) grade 2b and tumours with number >3 and size >5 cm, or tumours with number ≤3 and size >10 cm, as well as mALBI grade 1 or 2a and tumours with number >3 and size >10 cm, could serve as better indicators of TACE unsuitability in patients with intermediate-stage hepatocellular carcinoma.
- Within the framework of TACE unsuitability, the use of more precise discriminatory criteria is crucial to ensure that patients are not inappropriately excluded from the potential benefits of TACE.
- The integration of mALBI grade with the N3-S5-S10 tumour burden criteria may offer a practical framework for clinicians to individualise treatment selection, optimising outcomes by identifying patients more likely to benefit from TACE versus systemic therapy.
Introduction
Hepatocellular carcinoma (HCC) is one of the leading
malignancies worldwide. At diagnosis, up to 30% of
patients have intermediate-stage HCC according
to the Barcelona Clinic Liver Cancer system.1
Transarterial chemoembolisation (TACE) has
emerged as the first-line treatment for intermediate-stage
HCC, supported by two randomised controlled
trials2 3 and a meta-analysis4 that demonstrated
superior survival outcomes compared with best
supportive care or suboptimal therapies.
Because patients with intermediate-stage HCC
comprise a heterogeneous group characterised by
a wide range of tumour burdens and liver function,
the effectiveness of TACE as first-line treatment may
not be universal, particularly in subgroups with high
tumour burden or suboptimal liver function. To
address this issue, sub-staging of intermediate-stage
HCC based on tumour burden and liver function
has been proposed in several criteria, including
the Bolondi,5 Kinki,6 and MICAN (Modified
Intermediate Stage of Liver Cancer) criteria.7
These criteria have demonstrated discriminative prognostic value in identifying subgroups of patients
with intermediate-stage HCC.7 8 Given that survival
outcomes of patients treated with TACE can vary
across substages of intermediate-stage HCC, it is
clinically essential to identify thresholds of tumour
burden and liver function that preclude the use of
TACE according to survival benefit.
Sorafenib has been established as the standard
of care for advanced HCC since 2007, based on the
demonstration of its significant survival superiority
over placebo.9 10 11 Subgroup analyses of clinical
trials have shown that sorafenib exerts positive
therapeutic efficacy in intermediate-stage HCC, with
reported overall survival (OS) ranging from 14.5 to
20.6 months,9 12 13 which is comparable to the OS
achieved with TACE. Sorafenib treatment can serve
as a benchmark for evaluating the survival benefit
of TACE. If TACE does not provide a significant
survival benefit compared with sorafenib, it may not
be appropriate to subject patients to TACE rather
than systemic therapy, given that TACE is invasive
and potentially harmful to the liver. Patients may
benefit from systemic therapy before liver function
becomes suboptimal.
It has been hypothesised that specific baseline
parameters of tumour burden and liver function, at
which TACE fails to show superior survival benefit
compared with sorafenib, could be defined as
indicators of TACE unsuitability. This study aimed
to define specific indicators of TACE unsuitability
at baseline in patients with intermediate-stage HCC
according to thresholds of tumour burden and liver
function, as judged by the survival benefit of TACE
over sorafenib.
Methods
Study design
Due to the limited number of eligible participants,
all available cases with complete clinical data were
included. All patients with unresectable HCC who
received TACE or sorafenib therapy from January
2005 to December 2019 at Prince of Wales Hospital
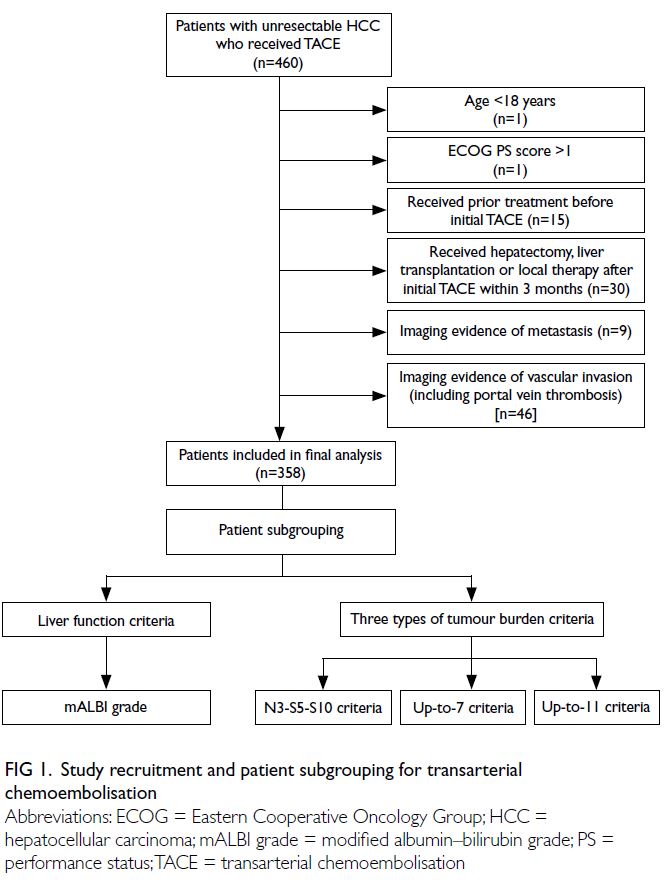
were enrolled in the study, provided they met all
eligibility criteria. Unresectability of intermediate-stage
HCC was determined by a multidisciplinary
team comprising a surgeon, an interventional
radiologist, and an oncologist. Inclusion criteria were
treatment-naïve, Barcelona Clinic Liver Cancer-B
stage HCC diagnosed by biopsy or a typical vascular
pattern on cross-sectional imaging; intrahepatic
disease without vascular invasion; and an Eastern
Cooperative Oncology Group performance status
score of 0 or 1. Exclusion criteria included age
under 18 years or Eastern Cooperative Oncology
Group performance status score of 2 or above;
prior treatment before initial TACE; receipt of
hepatectomy, liver transplantation, or local therapy after initial TACE; and any imaging evidence from
computed tomography (CT), magnetic resonance
imaging, or positron emission tomography/CT
showing vascular invasion by tumour (including
portal vein tumour thrombus) or extrahepatic
metastasis (Fig 1). To identify thresholds for TACE
unsuitability, OS of patients treated with TACE was
compared with that of patients treated with sorafenib
within subgroups defined by baseline tumour burden
and liver function. Overall survival was defined as the
interval between the initiation of TACE or sorafenib
and death from any cause. Patients who were alive or
lost to follow-up were censored.
Study participants
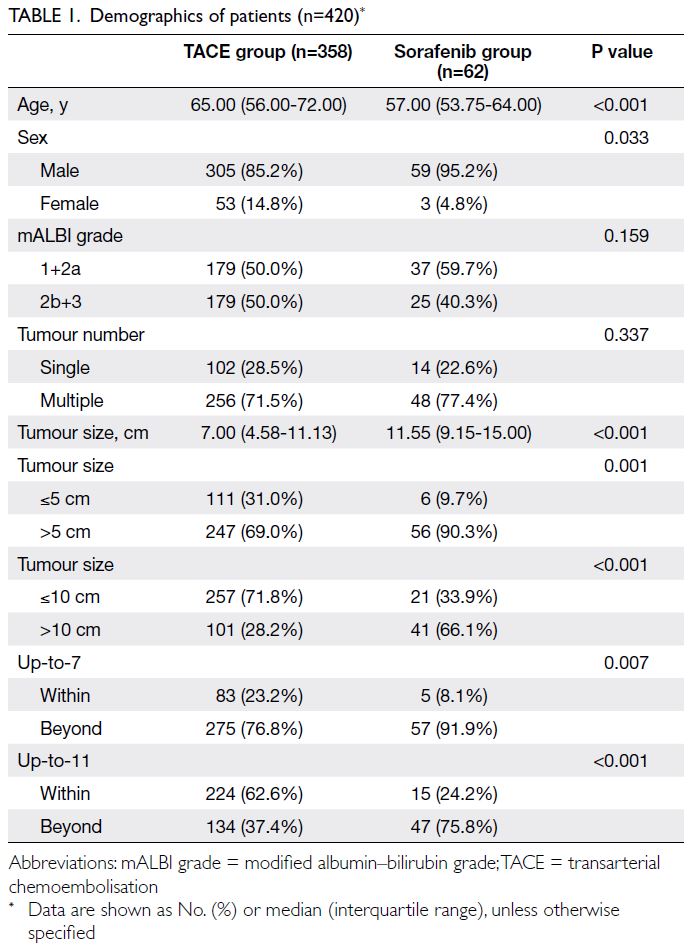
In total, 420 patients were enrolled in the study: 358
received TACE and 62 received sorafenib (Table 1).
The TACE group included significantly more older
and female patients. The median tumour size was
significantly larger in the sorafenib group compared
with the TACE group. No significant differences
were observed between the two groups in terms
of the modified albumin–bilirubin (mALBI) grade
distribution or tumour multiplicity. Among patients
initially treated with TACE, the median number of
TACE sessions was two (range, 1-4); 124 patients
received one session, 78 received two sessions, 53
received three sessions, and 103 received more than
three sessions. After developing refractoriness to
TACE, 60 patients subsequently received systemic
agents; of these, 35 received sorafenib, eight received
adriamycin, four received doxorubicin, six received
lenvatinib, and seven received other agents.
Patient subgrouping
Patients were classified into six subgroups according
to baseline tumour burden and liver function.
Tumour burden was subcategorised using the up-to-7, up-to-11, and N3-S5-S10 criteria. The up-to-7
and up-to-11 criteria were derived from the sum of
the maximum tumour size (in cm) and the tumour
number, with cut-off values of 7 or 11, respectively.
Accordingly, patients were categorised as within or
beyond the up-to-7 and up-to-11 criteria. In the N3-S5-S10 system, tumour burden was subcategorised
according to the combination of tumour number and
maximum tumour size; three tumour nodules and
5 cm or 10 cm in size served as the respective cut-off
values. This categorisation resulted in the following
six subgroups: (1) tumour number ≤3, tumour size
≤5 cm; (2) tumour number ≤3, tumour size >5 cm
to ≤10 cm; (3) tumour number ≤3, tumour size >10
cm; (4) tumour number >3, tumour size ≤5 cm; (5)
tumour number >3, tumour size >5 cm to ≤10 cm; and
(6) tumour number >3, tumour size >10 cm (Fig 1).
Liver function subgroups were classified
according to the mALBI grade.14 The mALBI grades
were determined using the ALBI score, calculated as (log10 [bilirubin level (μmol/L)] × 0.66) + (albumin
level [g/L] × –0.085). Based on three cut-off ALBI
scores, grades were defined as follows: grade 1
(≤–2.60), grade 2a (>–2.60 to ≤–2.27), grade 2b
(>–2.27 to ≤–1.39), and grade 3 (>–1.39). Because
the sample size of patients receiving sorafenib with
mALBI grade 1 or 2a was relatively small, these two
subgroups were combined for analysis. Additionally,
given that no patient with mALBI grade 3 received
sorafenib, this subgroup was excluded from the
analysis (Fig 1).
Transarterial chemoembolisation
The TACE procedures were performed using
digital subtraction angiography equipment via
a femoral approach under local anaesthesia.15 16
In brief, a microcatheter was used to catheterise
tumour-feeding arteries at the lobar, segmental,
or subsegmental level, depending on tumour size.
An emulsion of cisplatin–ethiodised oil (Platosin;
Pharmachemie BV, Haarlem, the Netherlands),
consisting of up to 20 mg aqueous cisplatin (20 mL) and up to 20-mL ethiodised oil mixed in a 1:1 volume
ratio, was administered until flow stasis occurred or
a maximum dose of 40-mL emulsion was delivered.
Digital subtraction angiography, with or without
non-contrast multiplanar CT, was used to confirm
treatment completeness. A gelatin sponge (5-10 mL)
was used to embolise the feeding arteries.
Postprocedure monitoring included blood
tests for liver function and tumour markers within
2 days, at 2 weeks, and then every 1 to 3 months, as
well as CT imaging every 3 months. Systemic therapy
was administered to patients with well-preserved
liver function who developed TACE refractoriness,
as indicated by continuous elevation of tumour
markers and CT evidence of tumour progression.
Systemic therapy
According to the customary protocol at Prince
of Wales Hospital, The Chinese University of
Hong Kong during the study period, patients with
unresectable intermediate-stage HCC and no contraindications to TACE were prioritised for
TACE treatment. Patients who declined TACE were
treated with sorafenib; as a result, some patients in
the sorafenib group had smaller tumours or fewer
tumour nodules. Sorafenib was administered orally
at a prescribed dose of 400 mg twice daily. In the
event of intolerable side-effects or serious adverse
events, oncologists could adjust the treatment by
reducing the dose or discontinuing the drug.
Statistical analysis
Categorical variables were presented as numbers
(percentages), while continuous variables were
summarised as median (interquartile range), median
(95% confidence interval [95% CI]), or depending
on the results of normality testing. The Chi squared
test was used to compare categorical data, and the
Mann-Whitney U test was performed for continuous
data. Kaplan-Meier curves and Cox proportional
hazards models were used to compare OS values
among subgroups. The log-rank test and hazard
ratio (HR) were utilised to assess survival differences
between subgroups. A sensitivity analysis of survival
outcomes was conducted, excluding participants
who received systemic therapy after TACE. A P
value <0.05 was considered statistically significant.
Statistical analyses were performed using SPSS
(Windows version 25.0; IBM Corp, Armonk [NY],
United States).
Results
Comparison of overall survival between
transarterial chemoembolisation and sorafenib
The median OS of all patients who received TACE
was significantly longer than that of patients who
received sorafenib (19.37 [16.89-21.85] months vs
5.12 [4.37-5.84] months, P<0.001; Fig 2a). When
stratified by mALBI grade, patients with mALBI
grade 1 or 2a had significantly longer median OS in
the TACE group compared with the sorafenib group
(23.83 [18.53-29.13] months vs 6.60 [3.61-9.59]
months, P<0.001; Fig 2b). Similarly, patients with
mALBI grade 2b had significantly longer median
OS in the TACE group than in the sorafenib group
(16.20 [11.91-20.49] months vs 4.39 [3.44-5.35]
months, P<0.001; Fig 2c).
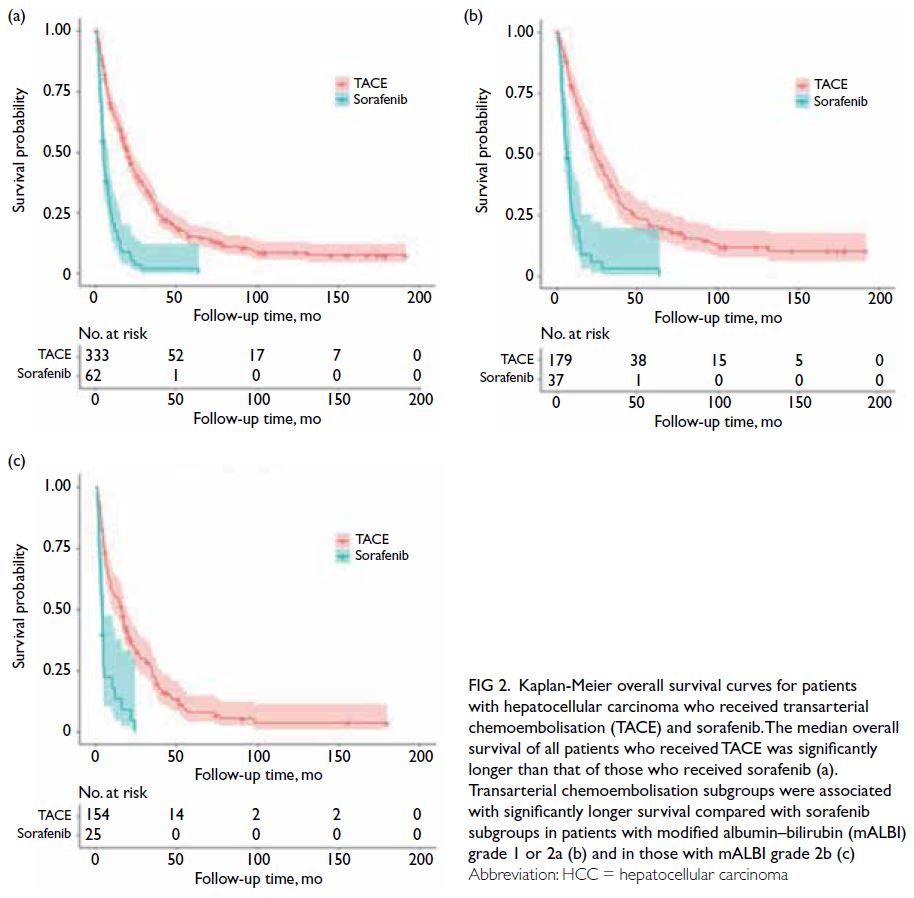
Figure 2. Kaplan-Meier overall survival curves for patients with hepatocellular carcinoma who received transarterial chemoembolisation (TACE) and sorafenib. The median overall survival of all patients who received TACE was significantly longer than that of those who received sorafenib (a). Transarterial chemoembolisation subgroups were associated with significantly longer survival compared with sorafenib subgroups in patients with modified albumin–bilirubin (mALBI) grade 1 or 2a (b) and in those with mALBI grade 2b (c)
Overall survival by modified albumin–bilirubin grade and tumour burden in
sorafenib-treated patients
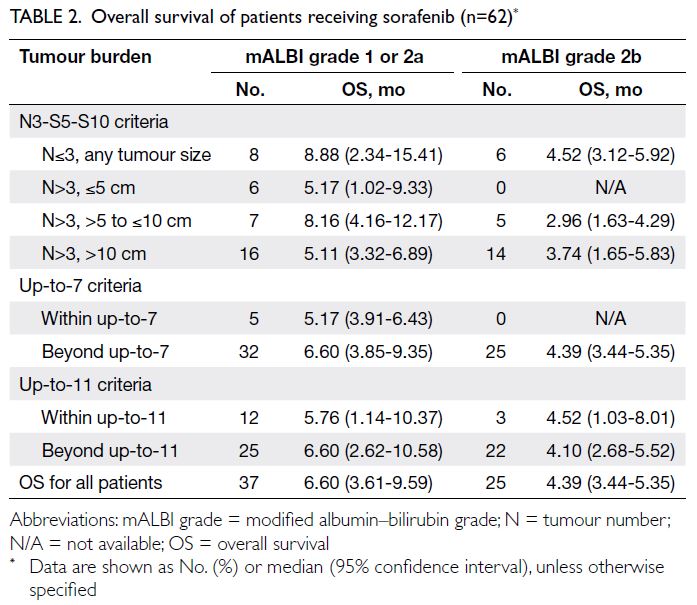
The median OS of patients treated with sorafenib,
stratified by mALBI grade and tumour burden, is
summarised in Table 2. As the sorafenib subgroups
with tumour number ≤3 had a relatively small
sample size (n=8) according to the N3-S5-S10
criteria, these patients were not further subdivided based on tumour size. Instead, they were combined
into a single subgroup with tumour number ≤3 to
increase the sample size for comparison with the
TACE group. Consequently, OS in the combined
sorafenib subgroup (tumour number ≤3, any tumour
size) was used for comparison with OS in the three
tumour-size TACE subgroups of tumour number ≤3
(Table 2).
The distribution of sample sizes was uneven
across the sorafenib subgroups with tumour number
>3 based on the N3-S5-S10 criteria, which may have
introduced bias in the survival outcomes, such as a
lower tumour burden being associated with worse
OS. To avoid underestimation of OS in any tumour-size
subgroup when comparing with the TACE
subgroups, the longest OS among the subgroups
with tumour number >3 was utilised as the OS value
for all these subgroups in the analysis, irrespective of tumour size (Table 2). As no patients with mALBI
grade 2 were present in the tumour burden subgroup
defined as within up-to-7, the OS of patients with
tumour burden beyond up-to-7 (Table 2) who were
treated with sorafenib was used as the control.
Overall survival in modified albumin–bilirubin grade 1 or 2a: transarterial chemoembolisation versus sorafenib
Table 3 presents the median OS of patients treated
with TACE or sorafenib, stratified by mALBI grade
1 or 2a and tumour burden. Across all subgroups
defined by various tumour burden criteria, patients
who received TACE achieved significantly longer
OS than those who received sorafenib (all P<0.05),
with HRs favouring TACE (ranging from 0.130 to
0.331). Sensitivity analysis showed that survival
was not significantly different between TACE and sorafenib in the subgroup with tumour number >3
and tumour size >10 cm (HR=0.418 [95% CI=0.147-1.171]; P=0.097).
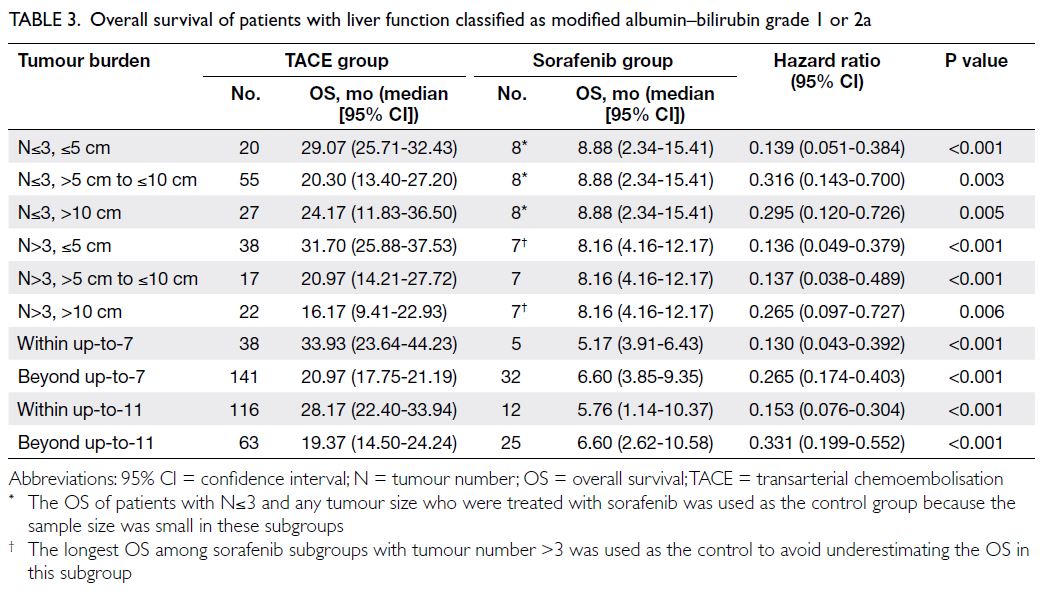
Table 3. Overall survival of patients with liver function classified as modified albumin–bilirubin grade 1 or 2a
Overall survival in modified albumin–bilirubin grade 2b: transarterial
chemoembolisation versus sorafenib
In subgroups with mALBI grade 2b, defined by either the up-to-7 or up-to-11 criteria, patients
who received TACE exhibited significantly longer
median OS than those who received sorafenib
across all subgroups (all P<0.05; Table 4). However,
when using the N3-S5-S10 criteria, TACE resulted
in a significantly longer median OS than sorafenib
only in the subgroups with tumour number ≤3 (any
tumour size) and in the subgroup with tumour
number >3 and tumour size ≤5 cm (both P<0.05;
Table 4). In the subgroups with tumour number
>3 and tumour size >5 cm to ≤10 cm, and those
with tumour number >3 and tumour size >10 cm,
although TACE subgroups demonstrated longer
median OS than sorafenib subgroups (6.07 vs 3.74
months and 7.73 vs 3.74 months, respectively), the
differences were not statistically significant (Table 4).
Sensitivity analysis showed that survival was also not
significantly different between TACE and sorafenib
in the additional subgroup with tumour number ≤3
and tumour size >10 cm (HR=0.474 [95% CI=0.185-1.261]; P=0.120).
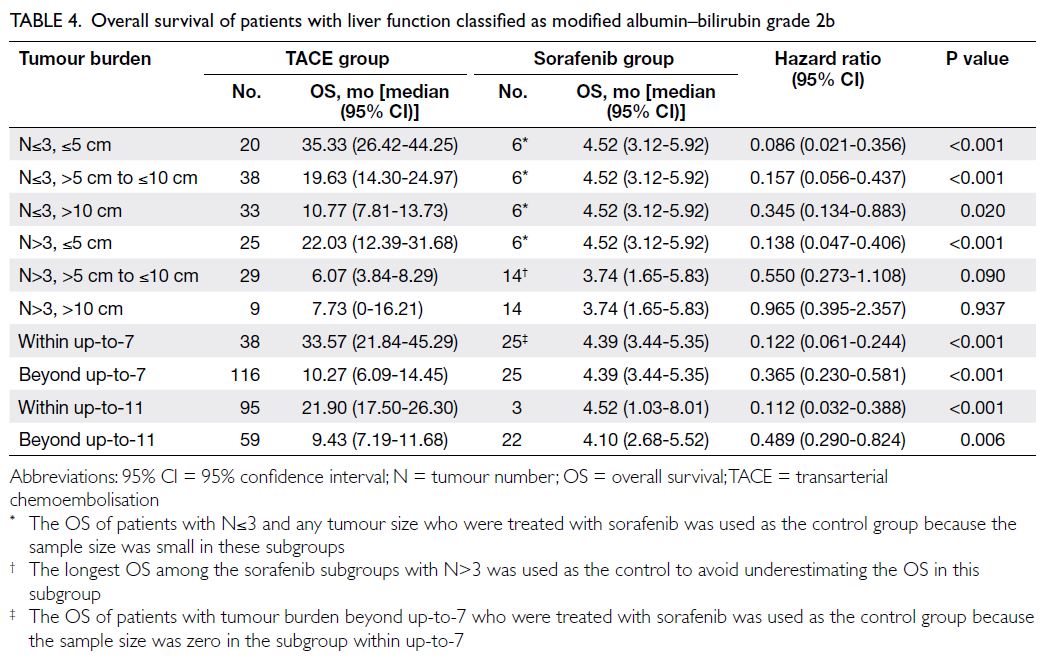
Table 4. Overall survival of patients with liver function classified as modified albumin–bilirubin grade 2b
Due to the small sample size, it was difficult
to demonstrate a clear survival benefit of TACE
over sorafenib; thus, the risk of overestimating the
survival benefit of TACE, due to potential bias from
more advanced disease in the sorafenib group, was
likely minimised. For example, given the limited
number of patients in the subgroups with tumour
number >3 and tumour size >5 cm to ≤10 cm and
those with tumour number >3 and tumour size >10
cm, these two subgroups were combined into one
subgroup (tumour number >3 and tumour size >5
cm). In this combined subgroup, TACE (n=38) still
yielded no significant survival benefit over sorafenib (n=14), with OS values of 6.07 months (4.10-8.03)
and 3.74 months (1.71-5.78), respectively (HR=0.586
[95% CI=0.325-1.054]; P=0.071).
Discussion
Results of subgroup analysis
Subgroup analysis in this study revealed that, within
the limitations of the data, TACE probably did not
confer a statistically significant survival benefit over
sorafenib for patients with mALBI grade 2b and a
high tumour burden (number >3 and size >5 cm,
or number ≤3 and size >10 cm), or for patients with
mALBI grade 1 or 2a and tumour burden of number
>3 and size >10 cm. In contrast, TACE did provide a
survival benefit when the beyond up-to-7 or beyond
up-to-11 criteria were applied. These findings
suggest that the use of more precise criteria to
define tumour burden and liver function could help
identify specific subgroups unsuitable for TACE.
Such criteria highlight the threshold at which
TACE no longer provides a survival advantage over
sorafenib, thereby indicating TACE unsuitability.
These indicators would be valuable in guiding
the clinical management of intermediate-stage
HCC. The small sample size in the sorafenib group
may have limited the statistical power to detect a
survival benefit of TACE in subgroups with tumour
number >3 and size >5 cm. Given that the overall results showed a consistent trend favouring TACE,
validation through further studies with larger sample
sizes is warranted.
Sorafenib as a control
In recent years, systemic therapy for HCC has
undergone rapid development, leading to the
emergence of new drugs after sorafenib. The
combination of certain agents has shown significant
improvements in survival compared with sorafenib
alone. The IMbrave150 study demonstrated that
treatment with atezolizumab plus bevacizumab
resulted in a significantly longer median OS than
sorafenib alone (19.2 vs 13.4 months).17 Similarly,
both sintilimab plus a bevacizumab biosimilar18
and tremelimumab plus durvalumab19 provided
significant survival benefits over sorafenib in
patients with unresectable HCC. Nevertheless,
sorafenib remains the first-line standard treatment
and the most effective single agent for advanced
HCC. It serves as a benchmark for newer single-agent
therapies such as lenvatinib, nivolumab, and
durvalumab, which have shown statistical non-inferiority
in survival compared with sorafenib.19 20 21
Therefore, the use of sorafenib as the control arm
versus TACE in this study is reasonable. With
the rapid advancement of systemic agents, novel
treatment strategies—such as switching to systemic
therapy22 or initiating systemic therapy upfront followed by curative conversion23—have been
advocated for patients with intermediate-stage HCC
who may not benefit from TACE or repeated TACE.
In such cases, it is important to define specific
indicators of TACE unsuitability among patients
with intermediate-stage HCC, in whom systemic
therapy may potentially improve survival.
Deficiencies of conventional criteria
of unsuitability for transarterial
chemoembolisation
The concept of TACE unsuitability has emerged in
conjunction with the development and availability of
systemic therapies.24 In patients with intermediate-stage
HCC, TACE unsuitability has been defined as
the presence of mALBI grade 2b and tumour burden
beyond the up-to-7 criteria.25 26 This definition was
based on worse survival in patients with mALBI
grade 2b and the beyond up-to-7 criteria relative to
patients displaying better liver function and lower
tumour burden, without addressing the potential
survival benefit of TACE over alternative treatment
options in this subgroup. However, this definition
has two key limitations. First, it lacks clinical
evidence demonstrating greater survival benefit
from other alternative treatments when TACE
is withheld. Second, there remains controversy
regarding the optimal criteria for defining high
tumour burden. If the beyond up-to-7 criteria is
used as the criterion for TACE unsuitability, the
majority of patients with intermediate-stage HCC
would be considered unsuitable, which is both
unrealistic and unsupported. In the present study,
79% of patients had high tumour burden beyond up-to-7, comparable to the 70% reported by Hung et al.27
Limitations of conventional sub-staging
systems
The sub-staging system using the up-to-11 criteria
has shown better discriminatory power than
the up-to-7 criteria for predicting survival after
TACE.28 29 Nonetheless, in this study, neither the
up-to-7 nor the up-to-11 criteria were able to
identify TACE unsuitability. The findings indicated
that both the patient subgroup with mALBI grade
2b and tumour burden beyond the up-to-7 criteria,
as well as the subgroup with mALBI grade 2b and
tumour burden beyond the up-to-11 criteria, still
derived survival benefits from TACE compared
with sorafenib, indicating that these subgroups
should not be considered TACE unsuitable. The
lack of discriminatory power may be attributed to
the persistently high heterogeneity among patients
classified as having high tumour burden under to
these two criteria. Worse survival after TACE in
these subgroups, compared with patients displaying
better liver function and lower tumour burden, does not justify entirely abandoning TACE in these patients.
We propose using the N3-S5-S10 criteria
to define tumour burden, as these criteria allow
for more specific subgrouping and enable the
identification of TACE unsuitability with greater
precision, thereby reducing the likelihood of denying
patients a potentially beneficial treatment (TACE).
Our findings demonstrate that the proposed criteria
can identify TACE unsuitability precisely in specific
subgroups where the up-to-7 or up-to-11 criteria
fail to distinguish survival differences. Based on
these findings, we recommend that physicians assess
intermediate-stage HCC using both the mALBI
grade and the N3-S5-S10 criteria—a more rigorous
framework—to determine TACE unsuitability. To
our knowledge, this is the first study to demonstrate
the survival benefit of TACE over sorafenib in
patients with intermediate-stage HCC stratified by
both liver function and tumour burden, as well as to
identify TACE unsuitability within these subgroups.
Limitations
This study provided a larger sample size than previous
studies comparing survival benefits between TACE
and sorafenib. However, several limitations should
be noted. First, the retrospective design of this study
inevitably introduced patient selection bias between
the TACE and sorafenib groups. Although there
were significant differences in age, sex, and tumour
size between the groups, such disparities in overall
patient demographics might not have critically
affected the validity of the survival comparisons,
given that these were based on subgroup analyses.
Second, the sample size was exceedingly small in
some sorafenib subgroups with low tumour burden.
The substantial disparity in patient numbers may
have contributed to non-significant differences in
OS between subgroups. We attempted to mitigate
this limitation by combining subgroups with very
small sample sizes. Third, some patients in the
TACE group received systemic therapy after disease
progression. Consequently, survival in the TACE
group may have been overestimated as it reflected
outcomes of TACE with or without systemic therapy,
rather than TACE alone. Nonetheless, ‘TACE
followed by systemic therapy’ represents standard
clinical practice aimed at achieving the greatest
patient benefit, and isolating a TACE-alone group
for analysis would not be realistic. Notably, ‘TACE
followed by systemic therapy’ accurately reflects
real-world treatment practice and does not conflict
with the study’s primary objective, which was to
define specific indicators of TACE unsuitability
at baseline rather than at the point when TACE
becomes unsuitable. Finally, no power calculation
was performed in the statistical analysis.
Conclusion
More precise criteria for TACE unsuitability are
required. The combination of mALBI grade and
N3-S5-S10 criteria may serve as a better indicator
of TACE unsuitability than the beyond up-to-7
or beyond up-to-11 criteria for patients with
intermediate-stage HCC. TACE likely offers no
survival benefit compared with sorafenib beyond
these thresholds. However, validation in a larger
cohort is warranted.
Author contributions
Concept or design: SCH Yu.
Acquisition of data: LM Chen, L Li, EP Hui, W Yeo, SL Chan.
Analysis or interpretation of data: LM Chen, SCH Yu.
Drafting of the manuscript: LM Chen, SCH Yu.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: LM Chen, L Li, EP Hui, W Yeo, SL Chan.
Analysis or interpretation of data: LM Chen, SCH Yu.
Drafting of the manuscript: LM Chen, SCH Yu.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This research was funded by the Vascular and Interventional
Radiology Foundation, Hong Kong. The funding body was not
involved in the design of the study, collection of data, analysis/interpretation of data, or writing of the manuscript.
Ethics approval
This research was approved by The Chinese University of Hong
Kong–New Territories East Cluster Ethics Committee, Hong
Kong (Ref No.: 2020.672). It was conducted in accordance with
the Declaration of Helsinki and the International Conference
on Harmonisation–Good Clinical Practice guidelines. The
requirement for written informed patient consent was waived
by the Committee due to the retrospective nature of the
research.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics
2020: GLOBOCAN estimates of incidence and mortality
worldwide for 36 cancers in 185 countries. CA Cancer J
Clin 2021;71:209-49. Crossref
2. Llovet JM, Real MI, Montaña X, et al. Arterial embolisation
or chemoembolisation versus symptomatic treatment in
patients with unresectable hepatocellular carcinoma: a
randomised controlled trial. Lancet 2002;359:1734-9. Crossref
3. Lo CM, Ngan H, Tso WK, et al. Randomized controlled
trial of transarterial lipiodol chemoembolization for
unresectable hepatocellular carcinoma. Hepatology
2002;35:1164-71. Crossref
4. Llovet JM, Bruix J. Systematic review of randomized
trials for unresectable hepatocellular carcinoma:
chemoembolization improves survival. Hepatology Feb
2003;37:429-42. Crossref
5. Bolondi L, Burroughs A, Dufour JF, et al. Heterogeneity
of patients with intermediate (BCLC B) hepatocellular
carcinoma: proposal for a subclassification to facilitate
treatment decisions. Semin Liver Dis 2012;32:348-59. Crossref
6. Kudo M, Arizumi T, Ueshima K, Sakurai T, Kitano M,
Nishida N. Subclassification of BCLC B stage hepatocellular
carcinoma and treatment strategies: proposal of modified
Bolondi’s subclassification (Kinki criteria). Dig Dis
2015;33:751-8. Crossref
7. Hiraoka A, Kumada T, Nouso K, et al. Proposed new sub-grouping
for intermediate-stage hepatocellular carcinoma
using albumin–bilirubin grade. Oncology 2016;91:153-61. Crossref
8. Arizumi T, Ueshima K, Iwanishi M, et al. Validation of
Kinki criteria, a modified substaging system, in patients
with intermediate stage hepatocellular carcinoma. Dig Dis
2016;34:671-8. Crossref
9. Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety
of sorafenib in patients with advanced hepatocellular
carcinoma: subanalyses of a phase III trial. J Hepatol
2012;57:821-9. Crossref
10. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety
of sorafenib in patients in the Asia-Pacific region
with advanced hepatocellular carcinoma: a phase III
randomised, double-blind, placebo-controlled trial. Lancet
Oncol 2009;10:25-34. Crossref
11. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in
advanced hepatocellular carcinoma. N Engl J Med
2008;359:378-90. Crossref
12. Iavarone M, Cabibbo G, Piscaglia F, et al. Field-practice
study of sorafenib therapy for hepatocellular carcinoma:
a prospective multicenter study in Italy. Hepatology
2011;54:2055-63. Crossref
13. Marrero JA, Kudo M, Venook AP, et al. Observational
registry of sorafenib use in clinical practice across
Child-Pugh subgroups: the GIDEON study. J Hepatol
2016;65:1140-7. Crossref
14. Hiraoka A, Michitaka K, Kumada T, et al. Validation and
potential of albumin–bilirubin grade and prognostication
in a nationwide survey of 46,681 hepatocellular carcinoma
patients in Japan: the need for a more detailed evaluation
of hepatic function. Liver Cancer 2017;6:325-36. Crossref
15. Yu SC, Hui JW, Hui EP, et al. Unresectable hepatocellular
carcinoma: randomized controlled trial of transarterial
ethanol ablation versus transcatheter arterial
chemoembolization. Radiology 2014;270:607-20. Crossref
16. Yu SC, Hui JW, Li L, et al. Comparison of
chemoembolization, radioembolization, and transarterial
ethanol ablation for huge hepatocellular carcinoma (≥10
cm) in tumour response and long-term survival outcome.
Cardiovasc Intervent Radiol 2022;45:172-81. Crossref
17. Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety
data from IMbrave150: atezolizumab plus bevacizumab
vs. sorafenib for unresectable hepatocellular carcinoma. J
Hepatol 2022;76:862-73. Crossref
18. Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab
biosimilar (IBI305) versus sorafenib in unresectable
hepatocellular carcinoma (ORIENT-32): a randomised,
open-label, phase 2-3 study. Lancet Oncol 2021;22:977-90. Crossref
19. Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized,
open-label, multicenter study of tremelimumab (T) and
durvalumab (D) as first-line therapy in patients (pts)
with unresectable hepatocellular carcinoma (uHCC):
HIMALAYA. J Clin Oncol 2022;40(4_suppl):379. Crossref
20. Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib
in advanced hepatocellular carcinoma (CheckMate 459): a
randomised, multicentre, open-label, phase 3 trial. Lancet
Oncol 2022;23:77-90. Crossref
21. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib
in first-line treatment of patients with unresectable
hepatocellular carcinoma: a randomised phase 3 non-inferiority
trial. Lancet 2018;391:1163-73. Crossref
22. Ogasawara S, Ooka Y, Koroki K, et al. Switching to systemic
therapy after locoregional treatment failure: definition and
best timing. Clin Mol Hepatol 2020;26:155-62. Crossref
23. Kudo M. A novel treatment strategy for patients with
intermediate-stage HCC who are not suitable for TACE:
upfront systemic therapy followed by curative conversion.
Liver Cancer 2021;10:539-44. Crossref
24. Kudo M. Extremely high objective response rate of
lenvatinib: its clinical relevance and changing the
treatment paradigm in hepatocellular carcinoma. Liver
Cancer 2018;7:215-24. Crossref
25. Kudo M, Han KH, Ye SL, et al. A changing paradigm for the
treatment of intermediate-stage hepatocellular carcinoma:
Asia-Pacific Primary Liver Cancer Expert Consensus
Statements. Liver Cancer 2020;9:245-60. Crossref
26. Kudo M, Kawamura Y, Hasegawa K, et al. Management
of hepatocellular carcinoma in Japan: JSH Consensus
Statements and Recommendations 2021 update. Liver
Cancer 2021;10:181-223. Crossref
27. Hung YW, Lee IC, Chi CT, et al. Redefining tumor burden in
patients with intermediate-stage hepatocellular carcinoma:
the seven-eleven criteria. Liver Cancer 2021;10:629-40. Crossref
28. Kim JH, Shim JH, Lee HC, et al. New intermediate-stage
subclassification for patients with hepatocellular
carcinoma treated with transarterial chemoembolization.
Liver Int 2017;37:1861-8. Crossref
29. Lee IC, Hung YW, Liu CA, et al. A new ALBI-based model
to predict survival after transarterial chemoembolization
for BCLC stage B hepatocellular carcinoma. Liver Int
2019;39:1704-12. Crossref