Hong Kong Med J 2025;31:Epub 12 Sep 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
The scope and impact of original clinical research
by Hong Kong public healthcare professionals
Peter YM Woo, MMedSc, FRCS1,2; Desiree KK Wong, MB, BS1; Queenie HW Wong, MB, ChB1; Calvin KL Leung, MB, BS1; Yuki HK Ip, MB, BS1; Danise M Au, MB, BS1; Tiger SF Shek, MB, ChB1; Bertrand Siu, MB, BS1; Charmaine Cheung, MB, BS1; Kevin Shing, MB, BS1; Anson Wong, MB, ChB1; Yuti Khare, MB, BS1; Omar WK Tsui, MB, BS1; Noel HY Tang, BSc3; KM Kwok, MSc3; MK Chiu, MSc3; YF Lau, MPhil3; Keith HM Wan, MB, BS, FRCS4; WC Leung, MD, FRCOG5
1 Department of Neurosurgery, Kwong Wah Hospital, Hong Kong SAR, China
2 Department of Neurosurgery, Prince of Wales Hospital, Hong Kong SAR, China
3 Centre for Clinical Research and Biostatistics, The Chinese University of Hong Kong, Hong Kong SAR, China
4 Department of Orthopaedics and Traumatology, Kwong Wah Hospital, Hong Kong SAR, China
5 Department of Obstetrics and Gynaecology, Kwong Wah Hospital, Hong Kong SAR, China
Corresponding author: Dr Peter YM Woo (wym307@ha.org.hk)
Abstract
Introduction: This study reviewed the landscape
of clinical research conducted by public hospital
clinicians in Hong Kong. It also explored whether
an association exists between academic productivity
and clinical performance.
Methods: This was a territory-wide retrospective
study of peer-reviewed original clinical research
conducted by clinicians providing acute medical care
at non-university public hospitals between 2016 and
2021. Citations were retrieved from the MEDLINE
biomedical literature database. Scientometric
analysis was performed by collecting journal-level,
article-level, and author-level performance
indicators. Clinical performance was assessed
using crude mortality rate, inpatient hospitalisation
duration, and the number of 30-day unplanned
readmissions.
Results: In total, 3142 peer-reviewed studies were
published, of which 29.3% (n=921) were conducted
by non-university hospital public healthcare
professionals. The most productive specialty was
clinical oncology, with 0.56 articles published per
clinician. The overall mean journal impact factor
and Eigenfactor score were 2.34 ± 3.72 and 0.01 ±
0.07, respectively. At the article level, the mean total
number of citations was 6.33 ± 24.17, the mean Field
Citation Ratio was 3.37 ± 2.04, and the mean Relative
Citation Ratio (RCR) was 0.82 ± 3.32. A significant
negative correlation was observed between crude
mortality rate and RCR (r=-0.63; P=0.022). A
negative correlation was also identified between
30-day readmissions and RCR (r=-0.72; P=0.006).
Conclusion: Clinicians in Hong Kong’s public healthcare system are research-active and have achieved a substantial degree of influence in
their respective fields. Research performance was
correlated with hospital crude mortality rates and
30-day unplanned readmissions.
New knowledge added by this study
- More than 10% clinicians at non-university public hospitals in Hong Kong have engaged in original clinical research as principal investigators.
- In total, 29.3% of clinical research published in Hong Kong was conducted by professionals from non-university public hospitals.
- The quality of the research undertaken was encouraging. All medical specialties achieved a Field Citation Ratio greater than 1.00, indicating that their article citation rates exceeded those of counterparts in the same research field.
- Clinical research activity is correlated with reductions in hospital crude mortality rates and 30-day unplanned readmissions.
- The establishment of a research-supportive infrastructure and dedicated funding for non-university public hospitals may contribute to improved patient outcomes.
Introduction
Clinical research is fundamental to the advancement
of medicine. More than a quarter of a century on,
evidence-based medicine—which began as a nascent
movement in the early 1990s—has revolutionised
healthcare by producing trustworthy observations
that support better-informed clinical decision-making
and health policy.1 2 Research forms the
foundation of evidence-based medicine and
plays an important role in understanding disease,
thereby contributing to the development of novel
therapeutic strategies.3 This contribution has
translated into quantifiable outcomes: participation
in clinical research can lead to significant reductions
in patient mortality and inpatient length of stay
(LOS).4 5 6 7 8 9 Clinical research benefits individual
patients and drives socio-economic growth. The
UK National Institute for Health and Care Research
(NIHR) observed that every 1.0 GBP invested
in clinical trials yielded a return of up to 7.6 GBP
in economic benefit.10 However, a substantial
proportion of frontline clinicians typically do not
engage in research activities relevant to their daily
practice. A cross-sectional survey in North America
revealed that 32% of respondents did not know how
to participate in research.11 A similar study among
Hong Kong family physicians indicated that 27%
had no previous experience.3 Hong Kong is an ideal
location for conducting clinical research due to its world-class universal healthcare infrastructure,
electronic medical records system, use of English
in medical documentation, and the presence of a
pool of internationally reputable investigators.12 13
Additionally, the Hospital Authority (HA)—a
statutory body responsible for managing all public
hospitals in the city—provides more than 90%
of all inpatient bed-days, and the patient follow-up
rate is comparably high.14 Regardless of these
favourable factors, according to the Our Hong
Kong Foundation—a non-governmental, non-profit
public policy institute—the number of clinical trials
conducted in Hong Kong declined by 22% between
2015 and 2021, compared with a mean increase of
48% in developed countries and 285% in Mainland
China.15 No comprehensive review of the clinical
research activity of Hong Kong public healthcare
professionals has been conducted. Apart from
the UK and Spain, no other region has evaluated
the influence of clinician engagement in research
on key performance indicators within a universal
healthcare system.4 5 6 This study was performed to
determine Hong Kong’s research productivity in
terms of peer-reviewed published clinical studies,
its scholarly impact, and its influence on outcomes
for hospitalised patients—including LOS, crude
mortality, and 30-day unplanned readmission. A
comparative analysis of research productivity and
quality across medical disciplines was also performed.
Findings from this review could inform health policy
by providing a stronger foundation for the evidence-based
allocation of resources to support an efficient
and sustainable research ecosystem within the HA.
Methods
This was a territory-wide retrospective observational
study of peer-reviewed original clinical research
conducted by HA medical staff at general acute
care hospitals, in which the staff member served as
principal investigator. The review included articles
published in the biomedical literature from 1 January
2016 to 30 April 2021. Research articles from non-university
institutions—comprising 30% (13/43) of
all HA hospitals—were included. Citations covering
this 5.5-year period were retrieved from MEDLINE,
the United States National Library of Medicine’s
bibliographic database. The database was queried via
the PubMed Advanced Search Builder for all studies
published within the review period, where the first
author’s stated affiliation was a Hong Kong hospital.
The internet-based library search package RISmed
was used to extract author affiliation data from
the PubMed search results into R, an open-source
statistical software tool.16 17 The list of citations was
then manually reviewed to confirm that the study had
been conducted by a clinician from an HA hospital.
Published abstracts were categorised according
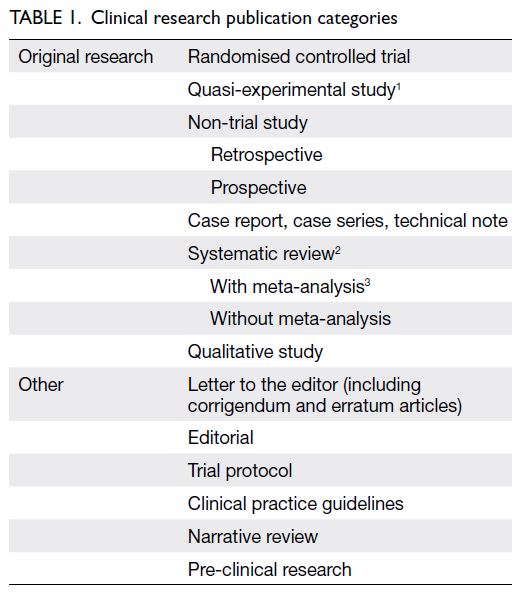
to study design, article type, and corresponding medical specialty (Table 1).18 Systematic reviews
performed in accordance with the PRISMA
(Preferred Reporting Items for Systematic Reviews
and Meta-Analyses) 2020 statement were regarded
as original research.19 20 Articles not subject to peer
review—such as academic conference proceedings,
trial protocols, editorials, letters to the editor, and
erratum or corrigendum statements—were excluded.
Preclinical studies and secondary research articles,
including clinical practice guidelines, position
statements, book chapters, and narrative topical
reviews, were also excluded. Finally, collaborative
studies in which the principal investigator was not
employed by the HA were excluded.
The primary study endpoint was research
productivity, measured by the total number of
original research studies published, with comparisons
made between university- and non–university-affiliated
HA hospitals. The hypothesis was that
university-affiliated hospitals would produce more
original clinical research studies than non-university
hospitals because of their access to tertiary education
institution resources. Secondary endpoints included
research productivity across medical specialties.
To control for workforce discrepancies across
medical disciplines, the mean number of full-time
clinicians for each specialty from 2016 to 2021 was
determined. The number of articles per clinician and
the proportion of the workforce acting as principal
investigator for each specialty were then established.
The quality of the research, as reflected by the
scientometric performance of each published article,
was also assessed. It was hypothesised that research
quality from university-affiliated hospitals would be
superior to that of their non-university counterparts. Another secondary endpoint was patient outcomes
for each non–university-affiliated acute care hospital
from 2016 to 2021: crude mortality rate per 100 000
hospitalised patients, length of inpatient stay, and
annual number of unplanned readmissions within 30
days of discharge. It was hypothesised that increased
research productivity would translate to improved
patient outcomes, and an inter-hospital comparison
of these key performance indicators was performed.
Patient outcome data were collected from the HA’s
Clinical Data Analysis and Reporting System and the
HA Management Information System.
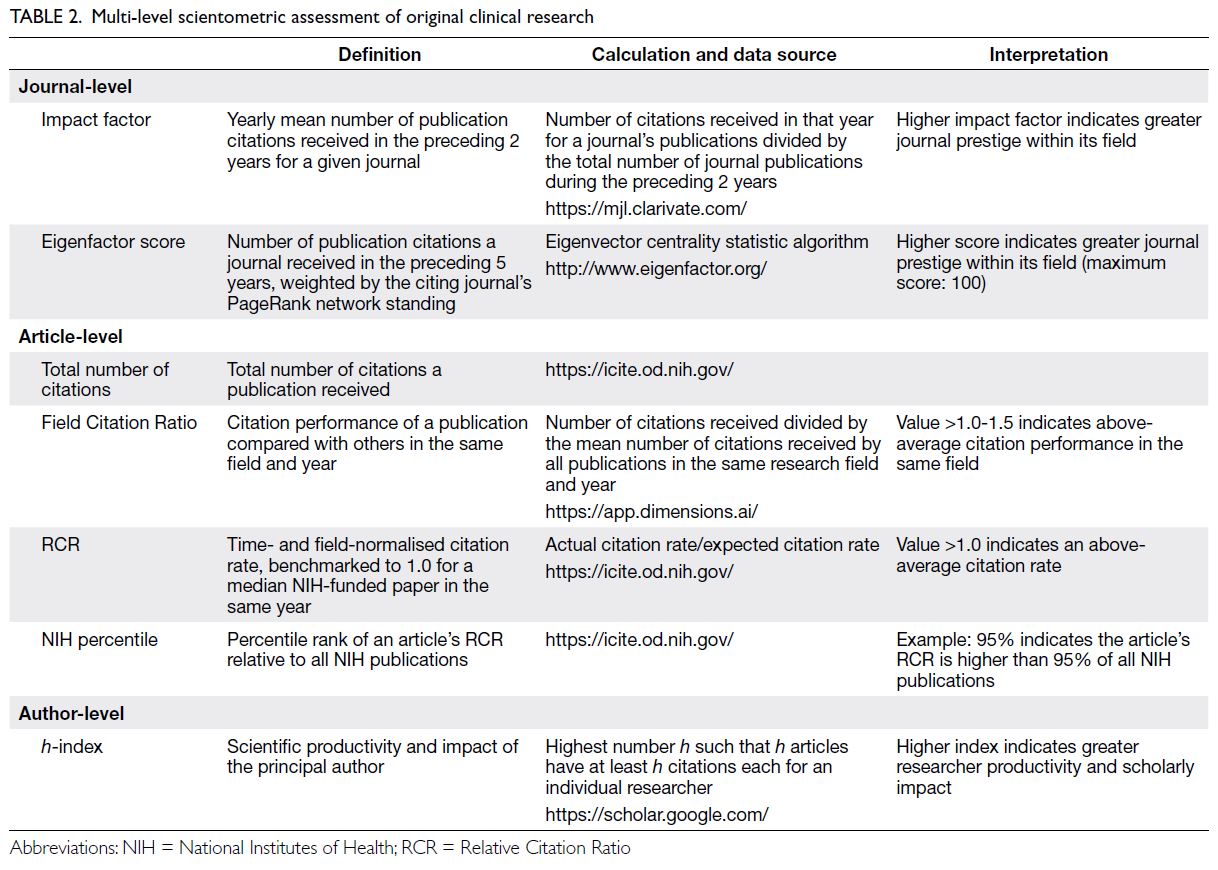
To evaluate research quality, a multi-level
scientometric approach was utilised by collecting
journal-, article-, and individual author-level data. For
journal-level scientometric assessment, two indices
were determined: the journal’s mean impact factor
(IF) and Eigenfactor score (ES) from 2016 to 2021.
These indices were obtained from Clarivate (London,
UK), a bibliometric analytics company that manages
the Science Citation Index, an online indexing
database containing academic journal citation data.1
A journal’s IF is a scientometric index reflecting the
mean number of citations received per article in that
journal during the preceding 2 years.21 This metric
constitutes a reasonable indicator of research quality
for general medical journals.22 The ES ranks journals
using eigenvector centrality statistics to evaluate the
importance of citations within a scholarly network.23
Utilising an algorithm similar to Google’s PageRank
(Alphabet Inc, Mountain View [CA], US), the ES
considers the number of citations received and
the prestige of the citing journal. For article-level
metrics, the total number of citations per article
(TNC), Relative Citation Ratio (RCR), Field Citation
Ratio (FCR), and National Institutes of Health (NIH)
percentile attained were documented (Table 2).
Author-level data were collected by determining the
h-index of the principal investigator (Table 2).24 All
scientometric data were censored on 30 June 2023.
Independent-samples t tests and Chi squared
tests were conducted to compare variables.
Spearman’s rank analysis was performed to assess
correlations between research and hospitalised
patient outcomes. P values of less than 0.05 were
considered statistically significant. All statistical
tests were performed using SPSS (Windows version
21.0; IBM Corp, Armonk [NY], US) and R (version
4.5.0; R Foundation, Vienna, Austria).17
Results
Overall original clinical research productivity
in Hong Kong
During the 5.5-year period, 4511 peer-reviewed
articles were published by Hong Kong medical
researchers from acute care public hospitals. Of
these, 3142 (69.7%) were original clinical research
studies. In total, 29.3% (n=921) of the articles were authored by non-university hospital investigators—a
significantly smaller proportion than that published by
their university hospital counterparts (independent-samples
t test, P<0.001) [Fig 1a]. Throughout the review period, the annual number of publications
by non-university hospital investigators remained
consistent, with a mean of 167 ± 8 per year (t test,
P=0.24) [Fig 1b]. Overall, the medical specialties that produced the most articles were internal medicine,
representing 23.4% (n=735) of published studies, and
general surgery, representing 16.5% (n=520) [Fig 2].
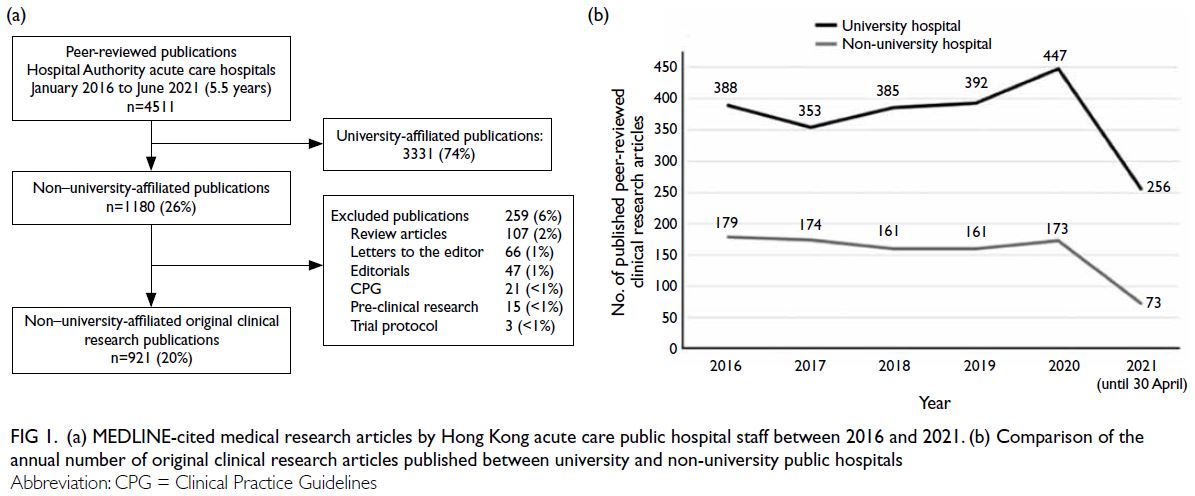
Figure 1. (a) MEDLINE-cited medical research articles by Hong Kong acute care public hospital staff between 2016 and 2021. (b) Comparison of the annual number of original clinical research articles published between university and non-university public hospitals
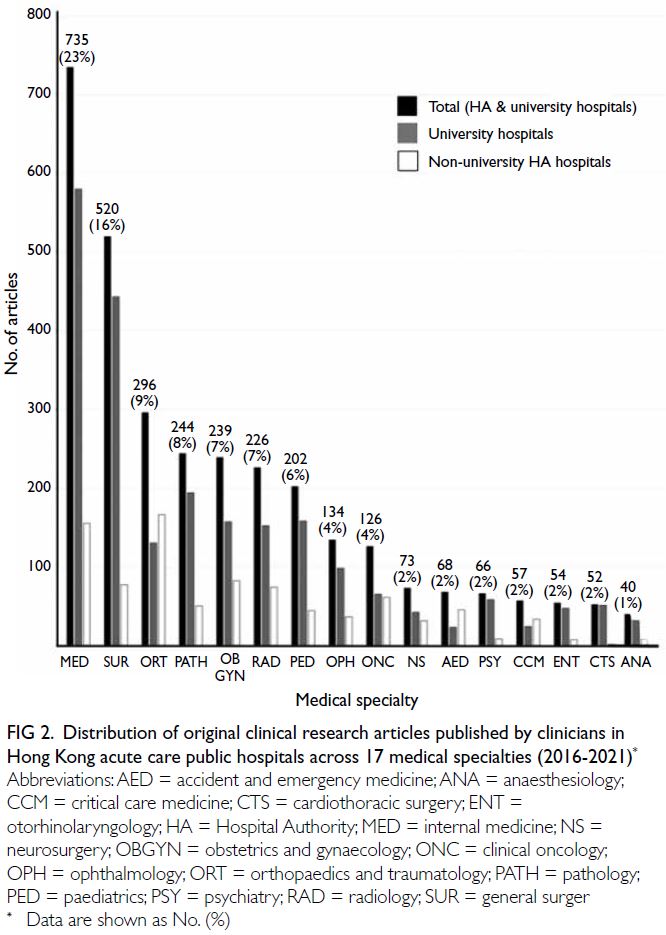
Figure 2. Distribution of original clinical research articles published by clinicians in Hong Kong acute care public hospitals across 17 medical specialties (2016-2021)
The majority of excluded articles were
narrative reviews that did not meet PRISMA criteria,
followed by letters to the editor and editorials
(Fig 1a). Regarding study design, most research
articles were case reports, followed by retrospective
cohort and prospective cohort studies (Fig 3).
A significantly larger proportion of case reports
were published by non-university hospital staff
compared with university hospital staff (P<0.001). In
addition to retrospective studies, university hospital
investigators were significantly more likely to publish
higher level-of-evidence research articles (Table 3).
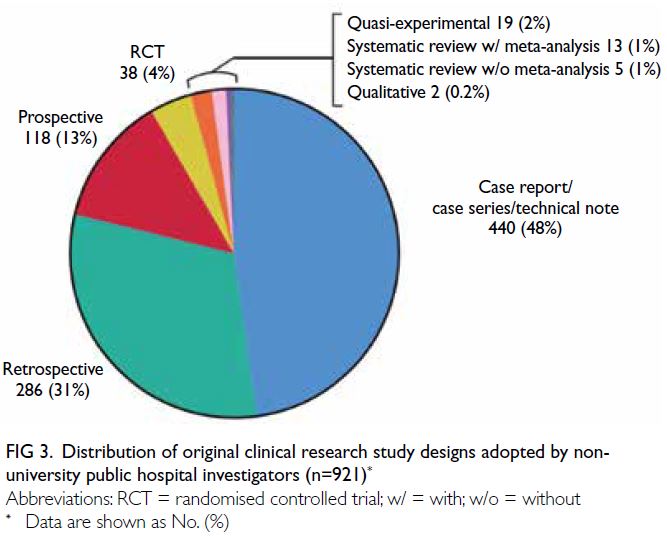
Figure 3. Distribution of original clinical research study designs adopted by non-university public hospital investigators (n=921)
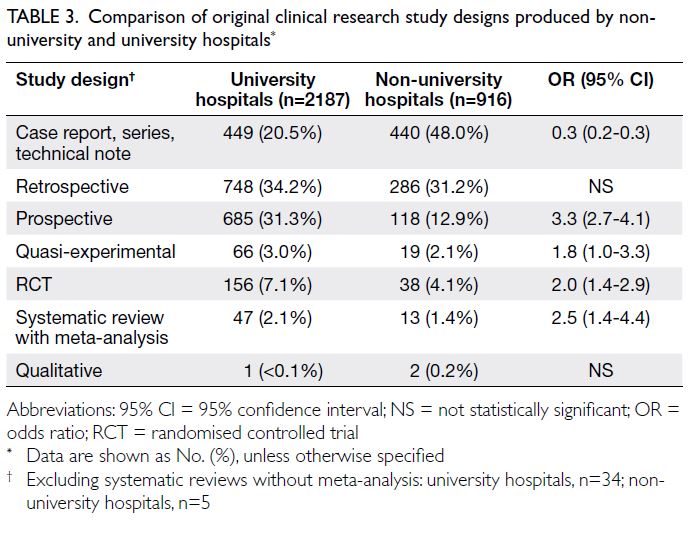
Table 3. Comparison of original clinical research study designs produced by non-university and university hospitals
Original clinical research productivity among
non-university general acute care hospitals and comparisons between medical specialties
The majority of non-university hospital principal
investigators were clinicians, with 3.7% (n=34) of
studies conducted by nurses or allied healthcare
professionals. Among the 887 articles authored
by clinicians, 544 individuals were identified,
yielding an author-to-article ratio of 1:1.6. These
researchers comprised 10.8% of the 5056 full-time
non-university hospital clinicians employed during
the study period. The most research-productive
specialties among non-university hospitals were
orthopaedics, followed by internal medicine and
obstetrics and gynaecology (Table 4). Among all the
medical specialties, the mean number of articles
per clinician (×100) was 17.5 ± 22.3 (range, 1.8-56.4). After controlling for workforce discrepancies
between disciplines, clinical oncology, orthopaedics
and traumatology, and obstetrics and gynaecology
constituted the most productive specialties in
terms of the mean number of articles published
per clinician (Table 4). Collectively, these three
specialties published significantly more studies
than the other disciplines (independent-samples
t test, P<0.001) [Table 4]. The most research-active
specialty was obstetrics and gynaecology, where one-third
of clinicians acted as principal investigators—this proportion was significantly larger relative to
other medical disciplines (t test, P=0.03) [Table 4].
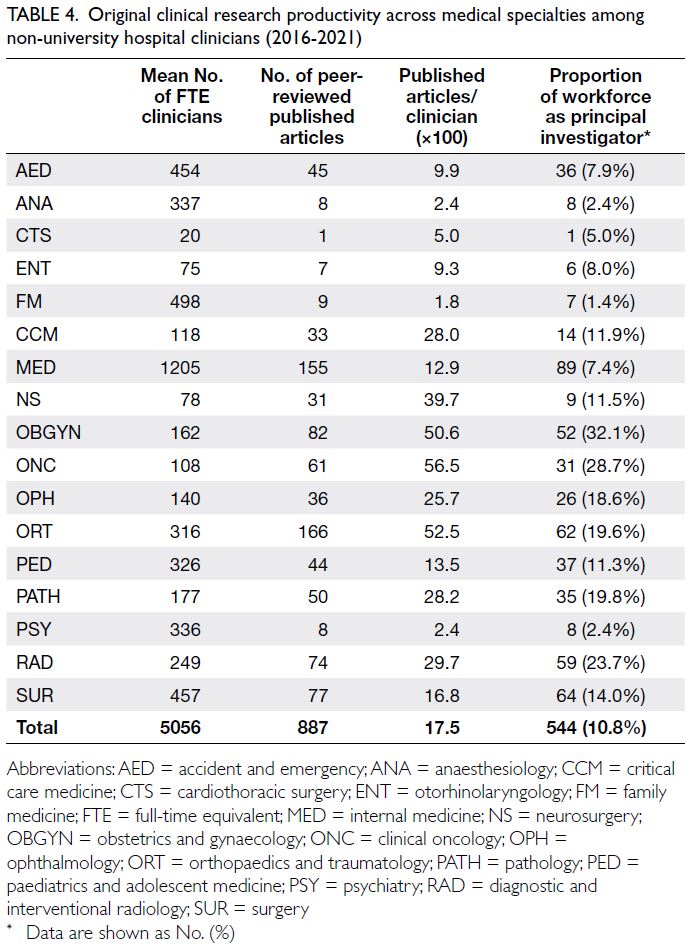
Table 4. Original clinical research productivity across medical specialties among non-university hospital clinicians (2016-2021)
Original clinical research quality among non-university general acute care hospitals and comparisons between medical specialties
Regarding scientometric performance, the overall
mean journal IF and ES were 2.34 ± 3.72 and
0.01 ± 0.07, respectively. No statistically significant
difference was observed between the principal
investigator’s medical specialty and the journal IF
(independent-samples t test, P=0.31). However, with
respect to the ES, clinical oncologists published
their research in journals with a significantly higher score relative to other medical disciplines (P<0.001) [Table 5].
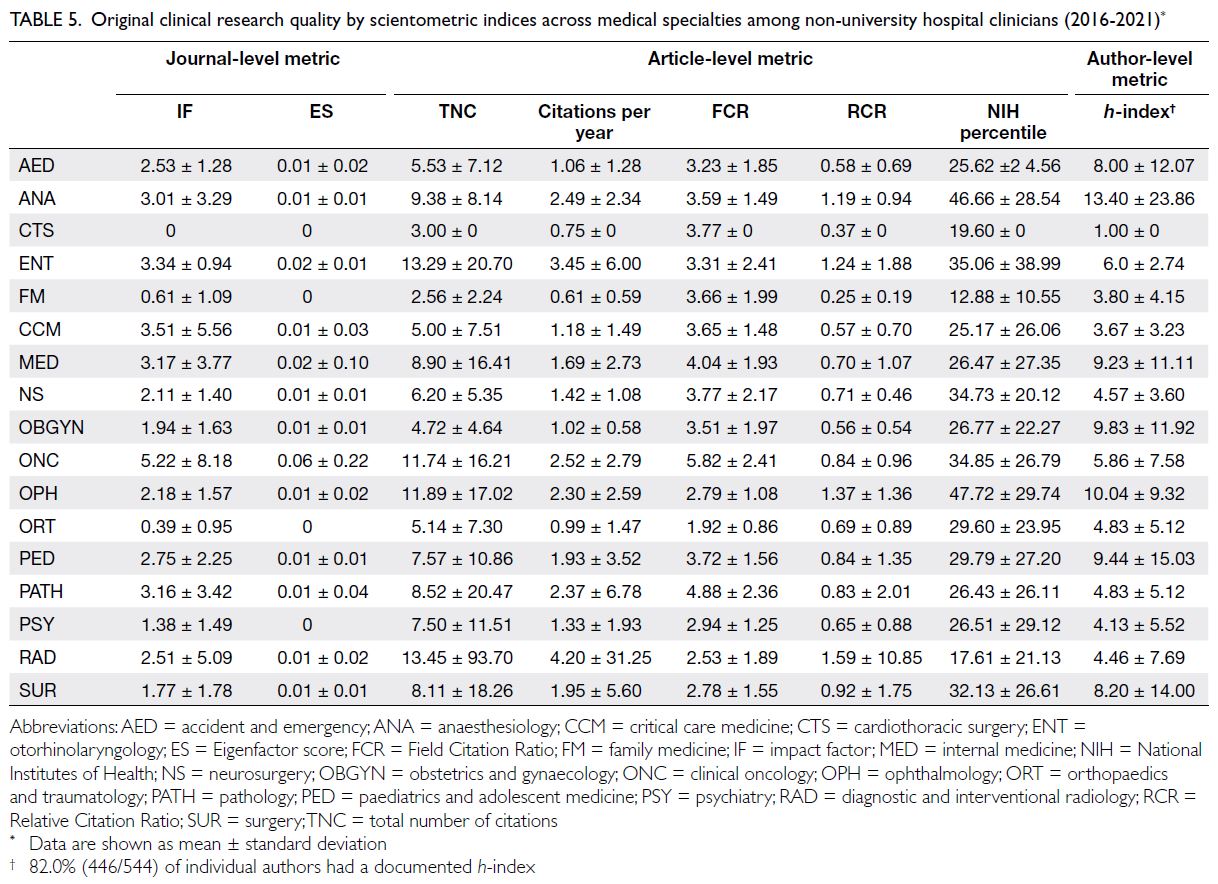
Table 5. Original clinical research quality by scientometric indices across medical specialties among non-university hospital clinicians (2016-2021)
For studies performed by non-university
clinicians during this period, at the individual
article level, the mean TNC per study was 6.33 ± 24.17, the mean number of citations per year was
1.81 ± 9.52, mean RCR was 0.82 ± 3.32, the mean
FCR was 3.37 ± 2.04, and the mean NIH percentile
achieved was 28.73 ± 25.85. Combined, radiology
and otorhinolaryngology research articles had a
significantly higher TNC per study (P<0.01) and a
higher total number of annual citations (P<0.001)
compared with other medical disciplines (Table 5). Articles in anaesthesiology, ophthalmology,
otorhinolaryngology, and radiology had a mean RCR
exceeding 1.00, indicating that their articles received
a higher citation rate than their co-citation network.
In particular, anaesthesiology and ophthalmology,
studies achieved the highest mean NIH percentile
rankings: their research outperformed 47% of
all NIH-associated publications. Combined,
anaesthesiology and ophthalmology studies also had
a significantly higher NIH percentile ranking than
other medical disciplines (P=0.001). All medical
specialties had an FCR exceeding 1.00, indicating
that their article citation rates were higher than
those of their counterparts in the same research field.
Oncology research had a significantly higher mean
FCR (5.82 ± 2.41) compared with other disciplines
(P<0.001) [Table 5].
In terms of author-level scientometric
performance, 18.0% (98/544) of authors did not
have a documented h-index. The mean h-index
for the remaining researchers was 7.54 ± 10.98.
Anaesthesiologists had a significantly higher
h-index relative to other specialties (independent-samples
t test, P=0.01) [Table 5]. A comparison of
scientometric outcomes between university and
non-university clinical research also demonstrated
uniformly superior performance by academic
institution investigators (Table 6).
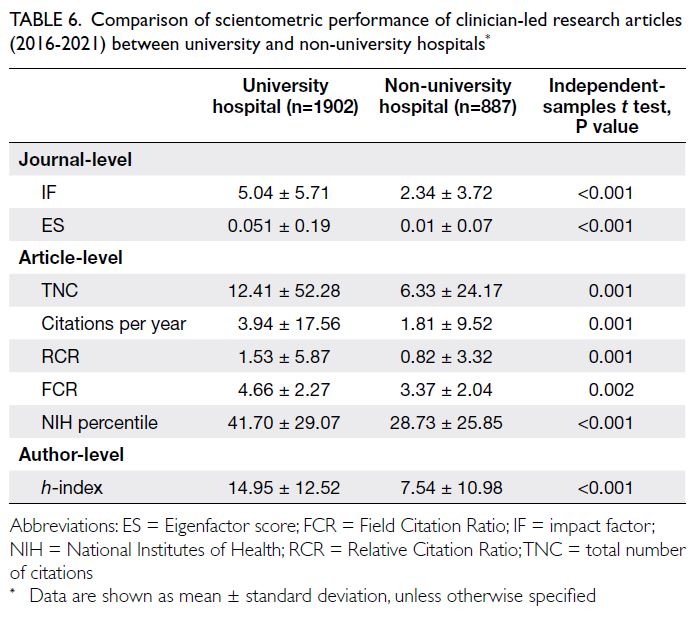
Table 6. Comparison of scientometric performance of clinician-led research articles (2016-2021) between university and non-university hospitals
Original clinical research and patient
outcomes
For non-university-affiliated hospitals, the overall
mean crude mortality rate per 100 000 hospitalised
patients was 27 722 ± 5208. Spearman’s rank
analysis identified significant negative correlations
of mortality rate with TNC (r=-0.69; P=0.01) and
RCR (r=-0.63; P=0.022). The overall annual mean
number of unplanned readmissions within 30 days
of discharge was 1408 ± 756. Similarly, there were
significant negative correlations of readmissions
with TNC (r=-0.76; P=0.02) and RCR (r=-0.72;
P=0.006). The overall mean LOS was 11.7 ± 3.1 days.
No significant correlations between LOS and TNC
(r=-0.32; P=0.29) or LOS and RCR (r=-0.36; P=0.23)
were detected. None of the other scientometric indices were associated with the crude mortality
rate, number of unplanned readmissions, or LOS.
Discussion
This study reviewed the breadth and quality of
original clinical research conducted by Hong Kong’s
public healthcare professionals. It is encouraging to
observe that, despite the heavy workload of frontline
clinicians employed in non-university public
hospitals, more than 10% of the workforce engaged
in original research as principal investigators. Their
endeavours contributed to nearly one-third of peer-reviewed
publications produced in the territory. A
multi-level scientometric approach was adopted to
assess research quality, and our findings indicate
that the studies undertaken met the standards
of their respective fields. Although the IF and ES
values of the published research were not high, all
medical specialties achieved a mean FCR of over
1.00. Notably, anaesthesiology, ophthalmology,
otorhinolaryngology, and radiology articles attained
an RCR exceeding 1.00.
Assessing research impact: the Relative
Citation Ratio
Introduced in 2016, the RCR is a relatively novel
article-level metric that measures a publication’s
relevance within the biomedical literature.24 It
was developed in response to the limitations
of conventional indicators of scientific quality,
such as the IF25 and h-index.26 For example, as
multidisciplinary collaborations have become
more common, researchers in disparate fields
may have unequal access to high-profile journals,
undermining the IF as a reliable reflection of a
study’s performance.21 24 25 Conversely, the h-index
does not consider an author’s total number of
citations and instead reflects cumulative output,
which can disadvantage early-career researchers.
Despite their limitations, the IF25 and h-index26
remain pivotal scientometric indices in decisions
related to funding and career progression. Given
that citations are widely recognised as a form of
acknowledging a researcher’s contribution to the
field, efforts have been made to formalise this
practice into a quantifiable metric. Endorsed by
the NIH, the RCR harnesses an article’s co-citation
network, normalising the number of citations
received according to the article’s publication time
and field of expertise. It is calculated as the ratio of
the article’s actual citation rate—derived from the
FCR—to the expected rate, benchmarked against
NIH-funded publications issued in the same year
and specialty.24 In recent years, the RCR has gained
recognition as a more reliable indicator of an article’s
performance within its peer comparison group and is
increasingly cited in research grant applications.27 28 29
Comparisons with university-affiliated
hospitals
The present study showed that university hospitals
not only outperformed non-university hospitals in
terms of research productivity, but also demonstrated
greater influence across all scientometric outcomes.
In addition to resource consolidation and the
employment of clinician-scientists, another reason
for this discrepancy might be the type of studies
produced. Approximately half of the articles from
non-university hospitals were case reports or
technical notes, which provide a lower level of
evidence in the evidence-based medicine hierarchy
and consequently tend to receive fewer citations.
Nonetheless, this form of research is more accessible
to junior clinicians and can serve as a gateway to
medical writing in resource-limited settings.30 Case
reports offer valuable insights into the real-world
implications of clinical practice—findings that well-designed
randomised controlled trials may fail to
capture. They can also stimulate others to report
similar observations, serving as a hypothesis-generating
opportunity for subsequent systematic enquiry.31
Translating research impact into real-world
patient outcomes
Few studies have tested the hypothesis that research
activity results in improved patient outcomes.4 5 7 8 32
We observed that non-university hospitals whose
staff engaged in clinical research had lower crude
mortality rates and annual 30-day unplanned
readmissions. These findings are supported by
reports that patients treated at hospitals participating
in clinical trials fared better in terms of 30-day
post-intervention mortality and overall survival,
relative to those treated at hospitals without such
arrangements. This trend has been observed for
conditions including acute myocardial infarction,
small-cell lung cancer, colorectal cancer, breast
cancer, and ovarian cancer.5 33 34 35 36 37 The possibility of
a trial effect was reinforced by a systematic review
of 13 studies, which attributed this phenomenon to
healthcare providers’ greater adherence to clinical
practice guidelines and their inclination to adopt
evidence-based practices.8 A subsequent systematic
review of 33 studies further demonstrated that
research activity improved healthcare system
performance—reflected by reductions in LOS and
risk-adjusted mortality, as well as improvements in
patient satisfaction.9 In contrast, few studies have
quantitatively analysed peer-reviewed scientometric
data and its relationship with patient outcomes. For
specific disease conditions, a negative correlation
was observed between acute myocardial infarction–related risk-adjusted mortality and a weighted
citations ratio among 50 Spanish hospitals.7 A
review of 147 National Health Service trusts in the
UK demonstrated a negative correlation between the
number of research article citations per admission
and standardised mortality ratios.5 Econometric
modelling using data from 189 Spanish hospitals
detected a significant reduction in LOS among
institutions that published more clinical research
articles or had a higher TNC per article.6
Encouraging public hospital healthcare
professionals to become principal investigators
There is increasing evidence that clinical research
engagement improves patient outcomes, but several
barriers to participation remain. First, clinicians
have demanding responsibilities that often prohibit
involvement in this time-consuming and resource-intensive
activity.38 39 40 Clinicians are under-recognised
for their overtime efforts—when such work is
typically undertaken—and are overburdened with
administrative procedures. Research-supportive
policies that provide protected time or incentivise clinicians through career advancement could help
foster a more scholastic environment.40 Second,
Hong Kong has a lengthy and duplicative clinical
trial approval process. In a survey of 250 clinician-researchers,
90% reported that approval for a phase I
first-in-human study certificate from the Hong Kong
SAR Government’s Department of Health required
over 3 months.15 Additionally, for HA Clinical
Research Ethics Committee study approvals, 50%
of respondents reported that the process typically
lasted more than 3 months, whereas multi-centre
trials frequently required over a year to begin
recruitment.15 The establishment of a primary review
authority for investigative drug registration—similar
to the United States Food and Drug Administration,
European Medicines Agency, or China’s National
Medical Products Administration—could help
streamline regulatory pathways. Third, most funding
agencies favour academician-led research over
community clinician-led efforts.38 For example, the
existing Hong Kong SAR Government’s Health and
Medical Research Fund and the Health Care and
Promotion Fund—with a combined annual budget
of US$530 million—have primarily been allocated
to academicians with access to robust research
infrastructure. The lack of financial support for
community hospitals to develop research capabilities
can have clinical implications.4 38 39 40 A review of
funding allocations from the NIHR revealed that
National Health Service trusts receiving relatively
lower levels of research funding had higher
risk-adjusted mortality.4 A survey of healthcare
professionals in Ontario, Canada, showed that 46%
were dissatisfied with their research involvement,
although 83% agreed it benefited their careers.39 The
major barriers identified were a lack of mentorship
and institutional stewardship.39 The establishment of
a clinical research institute and academy dedicated
to supporting early-career clinician-scientists
could help address these challenges.15 Modelled
after the NIHR, the provision of publicly funded
administrative services to accelerate translational
research—by facilitating grant applications for non–university-affiliated hospitals, offering biostatistical
support, training research support staff, and
nurturing partnerships in a multi-stakeholder
ecosystem—can be transformative.40 Following the
introduction of NIHR services, there was a tenfold
rise in publications, accompanied by a significant
increase in mean citation ratios.41 A survey of NIHR
stakeholders—including clinicians, nurses, and allied
health professionals—also revealed that its training
programmes enhanced their research capacity and
strengthened individual career development.42
Limitations
This study had several limitations. First, we retrieved studies only from the MEDLINE database and not
from other sources such as Scopus, Web of Science,
Google Scholar, PsycINFO (psychology), CINAHL
(nursing and allied health), or HMIC (healthcare
management, administration, and policy). MEDLINE
was selected because it is the only freely accessible
primary source for interrogating the biomedical
literature without requiring an institutional user
account. While MEDLINE focuses primarily
on medicine and the biomedical sciences, other
databases cover broader disciplines. Inclusion of
these databases would have been ideal, but resource
constraints prevented manual review for relevance.
Second, a comparison of patient outcomes between
university and non-university hospitals was not
performed as we were unable to determine whether
the principal investigator at teaching hospitals was
HA-employed or university-affiliated. Third, only
crude mortality rates and LOS were evaluated. A
more comprehensive review of public healthcare
system key performance indicators—such as risk-adjusted
or standardised mortality rates, symptom-to-intervention durations, incremental cost-effectiveness
ratios, and patient satisfaction survey
results—would have provided greater insight if
such data were available.6 43 Important confounding
factors were also not assessed, including each
hospital’s annual operational income; differences
in catchment population size and demographics;
and variations in the scope of acute clinical services
provided. For example, some institutions are
recognised as level-one trauma centres or infectious
disease centres. Finally, clinical research from
specialties such as psychiatry and family medicine
was likely under-represented, as most clinicians in
these fields work in dedicated psychiatric hospitals
or general outpatient clinics, which are outside the
scope of this study focused on general acute care
hospitals.
Conclusion
This study revealed that clinicians in Hong Kong’s
public healthcare system produced nearly one-third
of the original peer-reviewed clinical research
articles published from the territory. Although
the majority of these articles were case reports or
retrospective studies, they achieved a relatively high
degree of research influence within their respective
medical specialties. Research productivity appears
to be associated with improved patient outcomes,
particularly in terms of crude mortality rates and
30-day unplanned readmissions. Future studies
using more refined key performance indicator
endpoints and adjustments for confounding factors
are necessary to ascertain whether research-active
institutions consistently deliver better patient
outcomes.
Author contributions
Concept or design: PYM Woo, DKK Wong, YF Lau.
Acquisition of data: PYM Woo, DKK Wong, QHW Wong, CKL Leung, YHK Ip, DM Au, TSF Shek, B Siu, C Cheung, K Shing, A Wong, Y Khare, OWK Tsui, NHY Tang, KM Kwok, MK Chiu.
Analysis or interpretation of data: PYM Woo, DKK Wong.
Drafting of the manuscript: PYM Woo, DKK Wong.
Critical revision of the manuscript for important intellectual content: PYM Woo, DKK Wong, KHM Wan, WC Leung.
Acquisition of data: PYM Woo, DKK Wong, QHW Wong, CKL Leung, YHK Ip, DM Au, TSF Shek, B Siu, C Cheung, K Shing, A Wong, Y Khare, OWK Tsui, NHY Tang, KM Kwok, MK Chiu.
Analysis or interpretation of data: PYM Woo, DKK Wong.
Drafting of the manuscript: PYM Woo, DKK Wong.
Critical revision of the manuscript for important intellectual content: PYM Woo, DKK Wong, KHM Wan, WC Leung.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors thank Kwong Wah Hospital’s Clinical Research
Centre, The Hong Kong Student Association of Neuroscience
and the Hong Kong Olympia Academy for Clinical
Neuroscience Research for providing essential secretarial
support and assisting with data acquisition.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The research was approved by the Joint Chinese University
of Hong Kong–New Territories East Cluster Clinical Research
Ethics Committee, Hong Kong (Ref No.: 2025.256).
References
1. Djulbegovic B, Guyatt GH. Progress in evidence-based medicine: a quarter century on. Lancet 2017;390:415-23. Crossref
2. Ioannidis JP. Why most clinical research is not useful. PLoS
Med 2016;13:e1002049. Crossref
3. Chin WY, Wong WC, Yu EY. A survey exploration of the
research interests and needs of family doctors in Hong
Kong. Hong Kong Pract 2019;41:29-38.
4. Ozdemir BA, Karthikesalingam A, Sinha S, et al. Research
activity and the association with mortality. PLoS One
2015;10:e0118253. Crossref
5. Bennett WO, Bird JH, Burrows SA, Counter PR, Reddy VM.
Does academic output correlate with better mortality rates
in NHS trusts in England? Public Health 2012;126 Suppl
1:S40-3. Crossref
6. García-Romero A, Escribano Á, Tribó JA. The impact
of health research on length of stay in Spanish public
hospitals. Res Policy 2017;46:591-604. Crossref
7. Pons J, Sais C, Illa C, et al. Is there an association between
the quality of hospitals’ research and their quality of care? J
Health Serv Res Policy 2010;15:204-9. Crossref
8. Clarke M, Loudon K. Effects on patients of their healthcare
practitioner’s or institution’s participation in clinical trials:
a systematic review. Trials 2011;12:16. Crossref
9. Boaz A, Hanney S, Jones T, Soper B. Does the engagement
of clinicians and organisations in research improve
healthcare performance: a three-stage review. BMJ Open 2015;5:e009415. Crossref
10. National Institute for Health and Care Research. NIHR
Annual Report 2022/23. 2024. Available from: https://www.nihr.ac.uk/reports/nihr-annual-report-202223/34501. Accessed 18 Oct 2024.
11. Ciemins EL, Mollis BL, Brant JM, et al. Clinician
engagement in research as a path toward the learning
health system: a regional survey across the northwestern
United States. Health Serv Manage Res 2020;33:33-42. Crossref
12. Cheung BM, Yau HK. Clinical therapeutics in Hong Kong.
Clin Ther 2019;41:592-7. Crossref
13. Sek AC, Cheung NT, Choy KM, et al. A territory-wide
electronic health record—from concept to practicality:
the Hong Kong experience. Stud Health Technol Inform
2007;129:293-6.
14. Kong X, Yang Y, Gao J, et al. Overview of the health care
system in Hong Kong and its referential significance to
mainland China. J Chin Med Assoc 2015;78:569-73. Crossref
15. Our Hong Kong Foundation; Hong Kong Science and
Technology Parks. Developing Hong Kong into Asia’s
leading clinical innovation hub. November 2023.
Available from: https://ourhkfoundation.org.hk/sites/default/files/media/pdf/OHKF_BioTech_Report_2023_EN.pdf . Accessed 25 Oct 2024.
16. Kovalchik S. RISmed: download content from NCBI
Databases. R package version 2.3.0 2021. Available from:
https://CRAN.R-project.org/package=RISmed. Accessed 2 Jan 2023.
17. The R Foundation. R: a language and environment for
statistical computing Vienna, Austria; 2022. Available
from: https://www.R-project.org/. Accessed 2 Jan 2023.
18. Aquilina J, Neves JB, Tran MG. An overview of study
designs. Br J Hosp Med (Lond) 2020;81:1-6. Crossref
19. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA
2020 statement: an updated guideline for reporting
systematic reviews. Syst Rev 2021;10:89. Crossref
20. Krnic Martinic M, Meerpohl JJ, von Elm E, Herrle F,
Marusic A, Puljak L. Attitudes of editors of core
clinical journals about whether systematic reviews are
original research: a mixed-methods study. BMJ Open
2019;9:e029704. Crossref
21. Garfield E. The history and meaning of the journal impact
factor. JAMA 2006;295:90-3. Crossref
22. Suelzer EM, Jackson JL. Measures of impact for journals,
articles, and authors. J Gen Intern Med 2022;37:1593-7. Crossref
23. Bergstrom CT, West JD. Assessing citations with the
Eigenfactor metrics. Neurology 2008;71:1850-1. Crossref
24. Hutchins BI, Yuan X, Anderson JM, Santangelo GM.
Relative Citation Ratio (RCR): a new metric that uses
citation rates to measure influence at the article level. PLoS
Biol 2016;14:e1002541. Crossref
25. Casadevall A, Fang FC. Impacted science: impact is not
importance. mBio 2015;6:e01593-15. Crossref
26. Kreiner G. The slavery of the h-index—measuring the
unmeasurable. Front Hum Neurosci 2016;10:556. Crossref
27. Reddy V, Gupta A, White MD, et al. Assessment of the
NIH-supported relative citation ratio as a measure of
research productivity among 1687 academic neurological
surgeons. J Neurosurg 2020;134:638-45. Crossref
28. Didzbalis CJ, Avery Cohen D, Herzog I, Park J, Weisberger J,
Lee ES. The Relative Citation Ratio: a modern approach
to assessing academic productivity within plastic surgery.
Plast Reconstr Surg Glob Open 2022;10:e4564. Crossref
29. Gupta A, Meeter A, Norin J, Ippolito JA, Beebe KS. The
Relative Citation Ratio (RCR) as a novel bibliometric
among 2511 academic orthopedic surgeons. J Orthop Res
2023;41:1600-6. Crossref
30. Balinska MA, Watts RA. The value of case reports in
democratising evidence from resource-limited settings:
results of an exploratory survey. Health Res Policy Syst
2020;18:84. Crossref
31. Suvvari TK. Are case reports valuable? Exploring their role
in evidence-based medicine and patient care. World J Clin
Cases 2024;12:5452-5. Crossref
32. Selby P, Autier P. The impact of the process of clinical
research on health service outcomes. Ann Oncol 2011;22
Suppl 7:vii5-9. Crossref
33. Majumdar SR, Roe MT, Peterson ED, Chen AY, Gibler WB,
Armstrong PW. Better outcomes for patients treated at
hospitals that participate in clinical trials. Arch Intern Med
2008;168:657-62. Crossref
34. Rich AL, Tata LJ, Free CM, et al. How do patient and
hospital features influence outcomes in small-cell lung
cancer in England? Br J Cancer 2011;105:746-52. Crossref
35. Rochon J, du Bois A. Clinical research in epithelial ovarian
cancer and patients’ outcome. Ann Oncol 2011;22 Suppl
7:vii16-9. Crossref
36. Janni W, Kiechle M, Sommer H, et al. Study participation
improves treatment strategies and individual patient care
in participating centers. Anticancer Res 2006;26:3661-7.
37. Downing A, Morris EJ, Corrigan N, et al. High hospital
research participation and improved colorectal cancer
survival outcomes: a population-based study. Gut
2017;66:89-96. Crossref
38. DiDiodato G, DiDiodato JA, McKee AS. The research
activities of Ontario’s large community acute care hospitals:
a scoping review. BMC Health Serv Res 2017;17:566. Crossref
39. Senecal JB, Metcalfe K, Wilson K, Woldie I, Porter LA.
Barriers to translational research in Windsor Ontario: a
survey of clinical care providers and health researchers. J
Transl Med 2021;19:479. Crossref
40. Gehrke P, Binnie A, Chan SP, et al. Fostering community
hospital research. CMAJ 2019;191:E962-6. Crossref
41. Kiparoglou V, Brown LA, McShane H, Channon KM,
Shah SG. A large National Institute for Health Research
(NIHR) Biomedical Research Centre facilitates impactful
cross-disciplinary and collaborative translational research
publications and research collaboration networks: a
bibliometric evaluation study. J Transl Med 2021;19:483. Crossref
42. Burkinshaw P, Bryant LD, Magee C, et al. Ten years of
NIHR research training: perceptions of the programmes: a
qualitative interview study. BMJ Open 2022;12:e046410. Crossref
43. Jonker L, Fisher SJ, Dagnan D. Patients admitted to more
research-active hospitals have more confidence in staff and
are better informed about their condition and medication:
results from a retrospective cross-sectional study. J Eval
Clin Pract 2020;26:203-8. Crossref