Hong Kong Med J 2024;30:Epub 10 May 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Transurethral water vapour thermal therapy for benign prostatic hyperplasia under local anaesthesia alone: initial experience in Chinese patients
KL Lo, MB, ChB, FRCSEd; Alex Mok, MB, ChB; Ivan CH Ko, MB, ChB; Steffi KK Yuen, MB, BS, FRCSEd; Peter KF Chiu, MB, BS, FRCSEd; CF Ng, MD, FRCSEd
SH Ho Urology Centre, Department of Surgery, The Chinese University of Hong Kong, Hong Kong SAR, China
Corresponding author: Prof CF Ng (ngcf@surgery.cuhk.edu.hk)
Abstract
Introduction: This study evaluated the perioperative
and early postoperative outcomes of transurethral
water vapour thermal therapy (WVTT) under local
anaesthesia alone for benign prostatic enlargement
in Chinese patients.
Methods: This retrospective review of transurethral
WVTT for benign prostatic enlargement focused
on 50 Chinese patients who exhibited clinical
indications (acute retention of urine or symptomatic
lower urinary tract symptoms due to benign prostatic
enlargement) for surgical treatment between June
2020 and December 2021 in Hong Kong. Exclusion
criteria included active urinary tract problems and
urological malignancies. Follow-up was conducted
at 3 months postoperatively.
Results: The median patient age was 71.5 years. The
mean preoperative prostatic volume was 56.7 mL.
The mean operation time was 25.1 minutes. All
procedures were performed under local anaesthesia
alone. The mean pain scores for transrectal
ultrasound probe insertion, transperineal local
anaesthesia injection, and transurethral WVTT were
2, 5, and 4, respectively. Forty-nine patients (98%)
were discharged on the same day with a urethral
catheter. Forty-eight patients (96%) successfully
completed a trial without catheter within 3 weeks postoperatively. Five patients (10%) had unplanned
hospital admission within 30 days postoperatively
due to surgical complications (Clavien–Dindo grade 1).
Conclusion: Transurethral WVTT, an advanced
surgical treatment for benign prostatic enlargement,
is a safe procedure that relieves lower urinary tract
symptoms with minimal hospital stay. It can be
performed in an office-based setting under local
anaesthesia, maximising utilisation of the surgical
theatre.
New knowledge added by this study
- This is the first study concerning the efficacy and safety profile of water vapour thermal therapy (WVTT) in Asian patients. It can relieve lower urinary tract symptoms with minimal hospital stay.
- This is the first study of WVTT in an office-based setting under local anaesthesia, maximising utilisation of the surgical theatre.
- Water vapour thermal therapy is an effective and safe alternative for patients who have high surgical risk of benign prostatic enlargement under general or spinal anaesthesia.
Introduction
Benign prostatic hyperplasia (BPH) is characterised
by a non-malignant growth in the prostate gland
that can cause a wide range of lower urinary tract
symptoms (LUTS). These symptoms can greatly
reduce a patient’s quality of life (QoL) and may
eventually lead to acute retention of urine (AROU).
Current standard treatments for BPH include conservative, pharmacological, and surgical approaches. For patients who fail to successfully
complete a trial without catheter (TWOC) after
AROU secondary to BPH, surgical intervention
remains the main therapeutic approach. Surgical
treatment options for BPH have evolved from
electrosurgical resection to enucleation, ablation of
the prostate, and other techniques.1 Transurethral resection of the prostate (TURP), first performed
over 90 years ago, continues to be regarded as the
gold standard for the treatment of BPH with prostatic
volumes of 30 to 80 mL.2 Although TURP results in
statistically significant improvements in symptom
scores and maximum urinary flow rate (Qmax), it
has some limitations. Perioperative morbidities and
complications of TURP include infection, bleeding,
urinary retention, incontinence, urethral stricture,
erectile dysfunction, and ejaculatory dysfunction.
Additionally, TURP requires general or spinal
anaesthesia and postoperative hospitalisation.
Through technological advancements, several
minimally invasive procedures (eg, UroLift and
prostatic artery embolisation) have been developed
for specific groups of patients with BPH to minimise
the aforementioned limitations.3 4 5 Among these new-generation
BPH surgical approaches, transurethral
water vapour thermal therapy (WVTT) provides
some of the best surgical outcomes.
Transurethral WVTT uses the thermodynamic
principle of convective energy transfer, whereas
other techniques (eg, transurethral microwave
thermotherapy or transurethral needle ablation of
the prostate) involve conductive heat transfer.6 The
thermal therapy system consists of a generator with
a radiofrequency power supply that creates water
vapour from sterile water, as well as a disposable
transurethral delivery device. The tip of the delivery
device contains an 18-gauge needle with 12 small
emitter holes circumferentially arranged for water vapour dispersion into the targeted prostatic tissue.
The release of thermal energy causes tissue necrosis.
The most important characteristic of this technique
is that, during treatment of the transitional zone,
energy is only deposited within this specific region
of the prostate. Reviews of histological evidence
and magnetic resonance images have revealed that
thermal lesions are limited to the transitional zone
without affecting the peripheral zone, bladder,
rectum, or striated urinary sphincter.7 8 At 6 months
after treatment, the total prostatic volume is reduced
by 28.9% and the resolution of thermal lesions, as
determined by gadolinium-enhanced magnetic
resonance imaging, is almost complete.8
A pilot study showed that transurethral WVTT
can serve as a safe and effective treatment in men
with LUTS due to BPH.8 In the first multicentre,
randomised controlled study, 197 men were enrolled
and randomised in a 2:1 ratio to treatment with the
transurethral WVTT or a sham procedure.9 The
sham procedure consisted of rigid cystoscopy with
sound effects that mimicked the thermal treatment.
The primary efficacy endpoint was met at 3 months:
relief of symptoms, measured as a change in
International Prostate Symptom Score (IPSS), was
detected in 50% of patients in the thermal treatment
group compared with 20% of patients in the sham
procedure group (P<0.0001). In the thermal
treatment group, the Qmax increased by 63%—from
9.9 mL/s to 16.1 mL/s (P<0.0001)—after 3 months.
This clinical benefit was sustained throughout
the study period, with a 54% improvement at the
12-month follow-up. In the most recent update
regarding 5-year outcomes, the improvement in
voiding (as measured by IPSS and uroflowmetry)
had persisted for 5 years, with a surgical retreatment
rate of 4.4%.10
Thus far, studies of transurethral WVTT for
BPH have mainly focused on Caucasian populations.
To provide information regarding its tolerability
and effectiveness in the Chinese population, this
study investigated the safety profile and efficacy of
transurethral WVTT under local anaesthesia alone
for BPH among Chinese patients in Hong Kong.
Methods
Study protocol
This retrospective study investigated transurethral
WVTT for benign prostatic enlargement. The
inclusion criteria included Chinese ethnicity and
clinical indications for surgical treatment, including
AROU or symptomatic LUTS due to benign prostatic
enlargement. Exclusion criteria included active
urinary tract problems such as infection, bleeding
disorder, bladder pathologies (eg, bladder stones and
neurogenic bladder), and urethral stricture, as well
as urological malignancies including bladder and
prostate cancer.
Intervention
The procedure was performed with perioperative
antibiotic prophylaxis. Patients were placed in the
dorsal lithotomy position. After local anaesthesia,
cystoscopy was performed to assess the anatomy of
the bladder and prostate. A specialised handpiece
with an optical lens was inserted under direct visual
guidance into the prostate channel. Treatment began
with the needle tip visually positioned and inserted
approximately 1 cm distal to the bladder neck. Each
treatment lasted for 9 seconds. After 9 seconds,
an audible signal was produced by the system and
the treatment needle was retracted. The handpiece
was then repositioned 1 cm distal to the previous
treatment site; repositioning was repeated until
reaching a treatment site immediately proximal
to the verumontanum. During each water vapour
injection, the majority of the targeted tissue was
treated. All treatment cycles involving one lateral
lobe were completed as a group to utilise residual
heat from prior treatments involving that lobe.
Subsequently, the contralateral lateral lobe was
treated in a similar manner. An enlarged median
lobe could be treated by positioning the needle at a
45-degree angle towards the targeted lobe using the
same technique. After the procedure, a 14-Fr Foley
catheter was inserted. A 1-week course of antibiotic
treatment was administered after surgery.10 Patients
were discharged with the urethral catheter and
readmitted for a TWOC at approximately 1 to 2
weeks after surgery. Upon satisfactory completion of
the TWOC, patients were scheduled for follow-up at
3 months postoperatively.
Statistical analysis
Preoperative parameters and perioperative outcomes
were collected and tabulated using SPSS software
(Windows version 28.0; IBM Corp, Armonk [NY],
United States). Descriptive statistics were used to
summarise the demographic data and perioperative
patient characteristics. Paired sample t tests were
used to compare continuous variables with normal
distributions; the Mann-Whitney U test was used
to compare continuous variables with skewed
distributions, and the Chi squared test was used to
compare categorical variables. Two-sided P values of
<0.05 were considered statistically significant.
Results
Demographics
Between June 2020 and December 2021, 50 eligible
patients were included in this study. The median
age was 71.5 years (interquartile range [IQR]=64-75.25). In terms of indications, 27 patients (54%) had
symptomatic BPH, 13 patients (26%) had AROU with
a urethral catheter, and 10 patients (20%) had AROU
without a urethral catheter. Of the 50 patients, 39 (78%) were categorised as American Society of
Anesthesiologists class 2, whereas the remaining 11
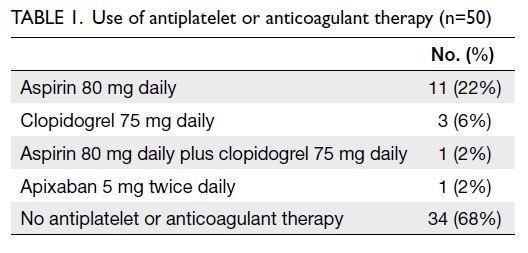
were categorised as class 3. Most patients (68%) did
not use any antiplatelet or anticoagulant therapy. The
numbers of patients using aspirin, clopidogrel, dual
antiplatelet therapy, and apixaban were 11 (22%),
three (6%), one (2%), and one (2%), respectively.
All antiplatelet and anticoagulant agents were
temporarily discontinued before the operation
(Table 1).
Operation
The mean preoperative prostatic volume was 56.7
mL (standard deviation [SD]=24.6; range, 29.2-119.0). The mean operation time was 25.1 minutes
(SD=8.4). All procedures were conducted under local
anaesthesia alone. Lignocaine 1% with adrenaline
was injected into the periprostatic space using a
transperineal approach. The mean pain scores for
transrectal ultrasound probe insertion, transperineal
local anaesthesia injection, and transurethral WVTT
were 2, 5, and 4, respectively.
Postoperative course
Only one patient (2%) required bladder irrigation for
5 days postoperatively; that patient had been taking
apixaban before surgery. All other patients were
discharged on the same day with a urethral catheter.
A TWOC was planned at around 1 week (for the
AROU without urethral catheter or symptomatic
BPH group) to 2 weeks (for the AROU with urethral
catheter group) after surgery. Forty-eight patients
(96%) in our study successfully completed a TWOC
within 3 weeks postoperatively; the median time was
7 days (IQR=7-14). The median successful TWOC
times were 14 days (IQR=8-21) for the AROU
with urethral catheter group and 7 days (IQR=7-12) for the AROU without urethral catheter or
symptomatic BPH group. Two patients (4%) with
an initially unsuccessful TWOC began temporary
clean intermittent self-catheterisation; they were
subsequently weaned from this management
approach on postoperative days 40 and 45,
respectively.
Five patients (10%) had unplanned hospital
admission within 30 days postoperatively due to
surgical complications (Clavien–Dindo grade 1). The reasons for readmission are listed in Table 2.
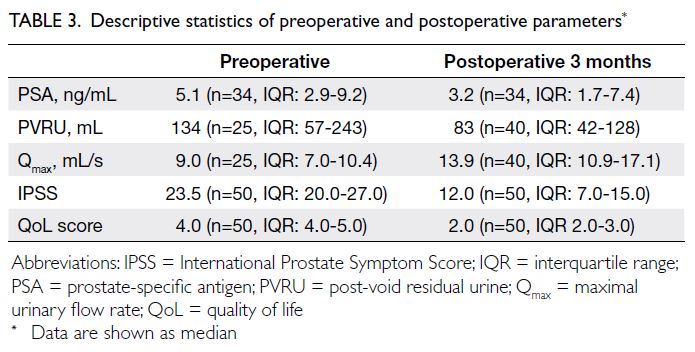
There were significant differences in
preoperative and 3-month postoperative parameters,
including prostate-specific antigen level, post-void
residual urine (PVRU) level, Qmax, IPSS, and QoL
assessment. Table 3 shows the medians and IQRs
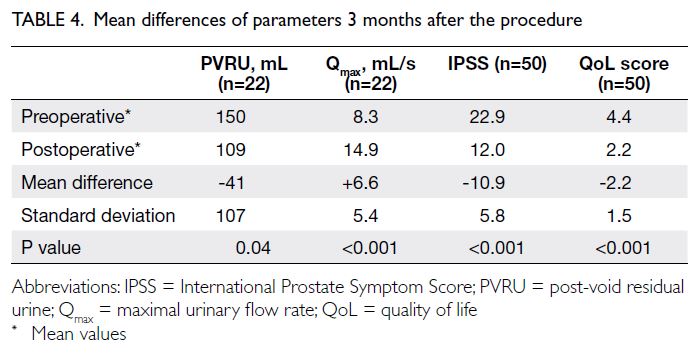
of these data. As indicated in Table 4, the mean
differences in PVRU, Qmax, IPSS, and QoL score were
-41 mL (SD=107), +6.6 mL/s (SD=5.4), -10.9 points
(SD=5.8), and -2.2 points (SD=1.5), respectively.
No patients in this study exhibited de novo
retrograde ejaculation or stress urinary incontinence
at 3 months postoperatively. However, there were
three reported cases (6%) of new-onset erectile
dysfunction postoperatively. All three patients
had temporary erectile dysfunction that resolved within 6 months postoperatively without requiring
medication.
Discussion
Our study is the first to focus on the application of
transurethral WVTT (Rezūm therapy) under local
anaesthesia alone among Chinese men with BPH.
Our results demonstrated clinically significant
outcomes comparable to other treatments for
BPH. Transurethral WVTT provided effective
symptomatic improvement, as illustrated by a
decrease in PVRU of 41 mL, an increase in Qmax of
6.6 mL/s, and a substantial decrease in IPSS of 10.9 at
the 3-month follow-up (Table 4). These results were
also comparable to outcomes in a recent international
study of this therapy.10 The postoperative outcome
was favourable, with a successful TWOC rate of
96% within 3 weeks postoperatively. Moreover,
all patients with urethral catheters before surgery
successfully completed a TWOC after transurethral
WVTT. The median successful TWOC time was
7 days postoperatively. However, compared with
data from other studies (4.1 to 5 days),11 12 our
centre had a longer duration of catheterisation,
which could be explained by our centre’s policy
of scheduling a TWOC on postoperative days 7
and 14 for patients without and with a urethral
catheter before surgery, respectively. Five patients
were readmitted within 30 days after surgery due
to haematuria, post-obstructive diuresis, recurrent
AROU, and urinary tract infection with AROU
(Table 2). All were uneventfully discharged without
further readmission; none of them developed
postoperative urinary incontinence. Three patients
reported de novo erectile dysfunction, higher than
the rate observed in the recent international study.10
However, the rate remained significantly lower
than that associated with TURP.13 Considering the
minimally invasive nature of this procedure, it could
revolutionise future management of BPH.
The current management algorithm for BPH
does not include transurethral WVTT as a first-line
treatment due to the relative lack of evidence
regarding its mid- to long-term efficacy and safety.2
However, it has considerable potential in the
management of BPH because of unique advantages compared with TURP. Transurethral WVTT
can be an office-based procedure with a short
learning curve. If a surgeon completes 10 cases of
transurethral WVTT under supervision, he/she will
become independent from a surgical trainer. Because
BPH is a particularly common urological disease and
often requires surgical management,14 the minimally
invasive nature of transurethral WVTT can help
reduce the number of patients waiting for operations
in overcrowded hospital facilities. The results of our
study provide initial evidence that transurethral
WVTT is well-tolerated among patients under
local anaesthesia alone. We did not administer any
sedation to the patients because they might move
during the operation, resulting in a high risk of
water vapour leakage. Such leakage would lead to
inadequate treatment.
In Hong Kong, total health costs represent
about 19% of the total government budget,15
and public in-patient health costs in 2021/2022
constituted 32% of total health costs.16 Operation
time is one of the most important factors affecting
in-patient costs. According to a meta-analysis by
Mamoulakis et al17 in 2009, the mean operation time
for TURP ranged from 39 to 79 minutes. In the present
study, the mean operation time was 25.1 minutes.
Thus far, no studies have directly assessed the cost-effectiveness
of transurethral WVTT in the Chinese
population. In the United States, a cost-effectiveness
analysis of six therapies for BPH, published in 2018,18
showed that transurethral WVTT was more cost-effective
than other minimally invasive therapies,
such as combination medical treatment and UroLift.
Moreover, McVary et al10 reported that the 5-year
retreatment rate after transurethral WVTT was
4.4%, which was significantly lower than that after
UroLift therapy (13.6%) reported by Roehrborn et al.5
Notably, transurethral WVTT leads to lower
incidences of bleeding, urgency, urge incontinence,
and ejaculatory dysfunction compared with TURP.19
The more favourable side-effect profile has resulted
in considerable interest concerning its potential to
replace TURP as the first-line surgical treatment in
the future. No head-to-head trials have compared
other surgical modalities with transurethral WVTT.
Indirect comparison through a meta-analysis
revealed that TURP outperformed transurethral
WVTT by providing greater relief of LUTS,19 although
it carried a greater cost and higher complication
rate.18 Although pharmacological treatment is
currently the first-line treatment for moderate to
severe LUTS, it is associated with complications such
as dizziness, postural hypotension, reduced libido,
and erectile dysfunction. Gupta et al20 compared
standard medical therapy with transurethral WVTT
using cohort data from the MTOPS trial (Medical
Therapy of Prostatic Symptoms); they showed that
transurethral WVTT had superior outcomes in terms of QoL, IPSS, and prostatic volume reduction.
Considering these advantages, transurethral WVTT
can be regarded as a first-line treatment option for
patients with symptomatic LUTS who prefer a short
operation, rather than lifelong pharmacological
treatment.
Limitations
There were some limitations in this study. First, a
substantial proportion of our patients had been
catheterised preoperatively (74%) and thus could
not undergo uroflowmetry studies before the
operation. Due to the coronavirus disease 2019
pandemic and the associated community isolation
policy, some other patients did not complete
uroflowmetry studies. However, all IPSS data were
able to be collected via telemedicine, ensuring the
inclusion of those data in the analysis. Second, our
study did not have a sufficient number of patients
to allow subgroup analysis of patients with different
indications for transurethral WVTT; future studies
should explore treatment outcomes among patients
with different indications for transurethral WVTT.
Third, our inclusion period was prolonged, partly
due to the coronavirus disease 2019 pandemic and
partly because transurethral WVTT mainly was
regarded as a self-financed item in our centre; these
aspects led to some difficulty in accumulating a
sufficient number of patients for analysis. Finally,
this study used a single-arm design with a relatively
short follow-up period; additional studies are
needed to assess long-term treatment outcomes and
retreatment rates after transurethral WVTT under
local anaesthesia alone.
Conclusion
Transurethral WVTT is a safe and effective treatment for benign prostatic hyperplasia in the
Chinese population. It can also be conducted in an
office setting under local anaesthesia alone, avoiding
use of the surgical theatre and its associated costs.
Author contributions
Concept or design: KL Lo, CF Ng.
Acquisition of data: KL Lo.
Analysis or interpretation of data: A Mok, ICH Ko.
Drafting of the manuscript: KL Lo, A Mok, ICH Ko.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: KL Lo.
Analysis or interpretation of data: A Mok, ICH Ko.
Drafting of the manuscript: KL Lo, A Mok, ICH Ko.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
As an editor of the journal, CF Ng was not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This research was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee, Hong Kong (Ref No.: 2019.662). The
patients were treated in accordance with the tenets of the
Declaration of Helsinki and have provided written informed
consent for all treatments and procedures and consent for
publication.
References
1. Kahokehr A, Gilling PJ. Landmarks in BPH—from aetiology to medical and surgical management. Nat Rev Urol 2014;11:118-22. Crossref
2. Gravas S, Cornu JN, Gacci M, et al. EAU guidelines on
management of non-neurogenic male lower urinary tract
symptoms (LUTS), incl. benign prostatic obstruction
(BPO). 2022. Available from: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Non-Neurogenic-Male-LUTS-2022.pdf. Accessed 8 May 2024.
3. Teoh JY, Chiu PK, Yee CH, et al. Prostatic artery
embolization in treating benign prostatic hyperplasia: a
systematic review. Int Urol Nephrol 2017;49:197-203. Crossref
4. Abt D, Hechelhammer L, Müllhaupt G, et al. Comparison
of prostatic artery embolisation (PAE) versus transurethral
resection of the prostate (TURP) for benign prostatic
hyperplasia: randomised, open label, non-inferiority trial.
BMJ 2018;361:k2338. Crossref
5. Roehrborn CG, Barkin J, Gange SN, et al. Five-year
results of the prospective randomized controlled prostatic
urethral L.I.F.T. study. Can J Urol 2017;24:8802-13.
6. Magistro G, Chapple CR, Elhilali M, et al. Emerging
minimally invasive treatment options for male lower
urinary tract symptoms. Eur Urol 2017;72:986-97. Crossref
7. Dixon CM, Rijo Cedano E, Mynderse LA, Larson TR.
Transurethral convective water vapor as a treatment for
lower urinary tract symptomatology due to benign prostatic
hyperplasia using the Rezūm(®) system: evaluation of acute
ablative capabilities in the human prostate. Res Rep Urol
2015;7:13-8. Crossref
8. Mynderse LA, Hanson D, Robb RA, et al. Rezūm system
water vapor treatment for lower urinary tract symptoms/benign prostatic hyperplasia: validation of convective
thermal energy transfer and characterization with magnetic
resonance imaging and 3-dimensional renderings. Urology 2015;86:122-7. Crossref
9. McVary KT, Gange SN, Gittelman MC, et al. Minimally
invasive prostate convective water vapor energy ablation:
a multicenter, randomized, controlled study for the
treatment of lower urinary tract symptoms secondary to
benign prostatic hyperplasia. J Urol 2016;195:1529-38. Crossref
10. McVary KT, Gittelman MC, Goldberg KA, et al. Final
5-year outcomes of the multicenter randomized sham-controlled
trial of a water vapor thermal therapy for
treatment of moderate to severe lower urinary tract
symptoms secondary to benign prostatic hyperplasia. J
Urol 2021;206:715-24. Crossref
11. Dixon CM, Cedano ER, Pacik D, et al. Two-year results
after convective radiofrequency water vapor thermal
therapy of symptomatic benign prostatic hyperplasia. Res
Rep Urol 2016;8:207-16. Crossref
12. Rowaiee R, Akhras A, Lakshmanan J, Sikafi Z, Janahi F.
Rezum therapy for benign prostatic hyperplasia: Dubai’s
initial experience. Cureus 2021;13:e18083. Crossref
13. Pavone C, Abbadessa D, Scaduto G, et al. Sexual
dysfunctions after transurethral resection of the prostate
(TURP): evidence from a retrospective study on 264
patients. Arch Ital Urol Androl 2015;87:8-13. Crossref
14. Lee YJ, Lee JW, Park J, et al. Nationwide incidence and
treatment pattern of benign prostatic hyperplasia in Korea.
Investig Clin Urol 2016;57:424-30. Crossref
15. Hong Kong SAR Government. The 2023-24 Budget.
Budget speech. Available from: https://www.budget.gov.hk/2023/eng/budget30.html. Accessed 5 May 2024.
16. Health Bureau, Hong Kong SAR Government. Hong Kong’s
domestic health accounts (DHA) 2021/22. Available from:
https://www.healthbureau.gov.hk/statistics/en/dha.htm. Accessed 5 May 2024.
17. Mamoulakis C, Ubbink DT, de la Rosette JJ. Bipolar
versus monopolar transurethral resection of the prostate:
a systematic review and meta-analysis of randomized
controlled trials. Eur Urol 2009;56:798-809. Crossref
18. Ulchaker JC, Martinson MS. Cost-effectiveness analysis
of six therapies for the treatment of lower urinary tract
symptoms due to benign prostatic hyperplasia. Clinicoecon
Outcomes Res 2018;10:29-43. Crossref
19. Tanneru K, Jazayeri SB, Alam MU, et al. An indirect
comparison of newer minimally invasive treatments for
benign prostatic hyperplasia: a network meta-analysis
model. J Endourol 2021;35:409-16. Crossref
20. Gupta N, Rogers T, Holland B, Helo S, Dynda D, McVary KT.
Three-year treatment outcomes of water vapor thermal
therapy compared to doxazosin, finasteride and
combination drug therapy in men with benign prostatic
hyperplasia: cohort data from the MTOPS trial. J Urol
2018;200:405-13. Crossref