Hong Kong Med J 2024 Feb;30(1):69–71 | Epub 8 Feb 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
High-dose N-acetylcysteine in an immunocompromised patient with COVID-19: a case report
KY Lai, FHKAM (Medicine), FRCP1; SY Au, FHKAM (Medicine), FRCP2; KC Sin, FHKAM (Medicine), FRCP1; SK Yung, FHKAM (Medicine), FRCP1; Anne KH Leung, FHKAM (Anaesthesiology), FJFICM1
1 Department of Intensive Care, Queen Elizabeth Hospital, Hong Kong SAR, China
2 Cardiovascular Centre, St Paul's Hospital, Hong Kong SAR, China
Corresponding author: Dr SY Au (h0145237@gmail.com)
Case presentation
On 19 June 2022, a 45-year-old man was admitted
to intensive care unit with severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2) pneumonia.
His body mass index was 22.3. He had a history of
hypertension, diabetes mellitus, hyperlipidaemia,
and immunoglobulin A nephropathy. He had been
receiving prednisolone and mycophenolate mofetil
following a renal transplant. His baseline creatinine
level was 203 μmol/L. Three doses of CoronaVac
vaccine had failed to induce an antibody response.
The patient was in septic shock and had
respiratory failure and acute renal failure. He had
myopericarditis with elevated level of serum troponin
I, diffuse ST elevation on electrocardiogram, and
impaired left ventricular ejection fraction of 40%
on echocardiogram. Coronary angiogram on 21
June 2022 was normal and myocardial biopsy
revealed increased interstitial macrophages. Urine
contained Enterococcus faecalis but no white blood
cells on microscopy. Screening for other bacterial,
viral or fungal co-infections was negative. He was
prescribed intravenous remdesivir 200 mg once
followed by 100 mg every 12 hours for four more
doses and intravenous hydrocortisone 100 mg
every 8 hours, and the nephrologist discontinued
his mycophenolate mofetil treatment. He received
broad spectrum antibiotics as directed by
infectious disease specialists. Enoxaparin 60 mg
was administered subcutaneously every 48 hours
for prophylaxis of deep vein thrombosis. Infectious
disease specialists did not recommend tocilizumab,
baricitinib, monoclonal antibodies, or convalescent
plasma.
The patient received intravenous high-dose
N-acetylcysteine at a dose appropriate for treatment
of paracetamol overdose as treatment of influenza-induced
cytokine storm. Animal studies have shown
that an oral dose of N-acetylcysteine 1 g/kg/day
improved the survival of mice with otherwise
lethal influenza infection and was synergistic with
oseltamivir with an endpoint survival of 100%.1
Human oral availability of N-acetylcysteine is 6%
to 10%. We have reported previously successful
treatment of a patient with 2009 H1N1 influenza virus pneumonia with N-acetylcysteine, administered as
a 100 mg/kg continuous intravenous infusion daily
for 3 days, with consequent suppression of fever and
C-reactive protein concentration and corresponding
clinical improvement.2 The C-reactive protein of this
patient reduced from 183 mg/L to 11.7 mg/L and
fraction of inspired oxygen requirement from 1.0 to
0.35. The positive end expiratory pressure reduced
from 18 cm H2O to 10 cm H2O. Nonetheless the
patient experienced a relapse of cytokine storm and
pulmonary deterioration following discontinuation
of high-dose N-acetylcysteine therapy before viral
clearance. The C-reactive protein rebounded to
132 mg/L and fraction of inspired oxygen
requirement increased to 0.85 and positive end
expiratory pressure requirement to 16 cm H2O.
Results for sepsis workup were negative. The patient
responded to reintroduction of N-acetylcysteine
therapy and showed no relapse of cytokine storm
when it was discontinued after viral clearance.2 The
patient, with SARS-CoV-2 pneumonia, received
an infusion of N-acetylcysteine 10 g in 500-mL 5%
dextrose solution at 21 mL/hour for 2 days from
21 June 2022. C-reactive protein level reduced from
278 mg/L to 72 mg/L and procalcitonin level from
>200 ng/mL to 33.33 ng/mL. Fraction of inspired
oxygen requirement decreased from 0.6 to 0.35
and positive end expiratory pressure requirement
from 10 cm H2O to 6 cm H2O over 2 days following
high-dose N-acetylcysteine infusion. When the
patient showed no signs of viral clearance on 26
June 2022 and antibodies against SARS-CoV-2
remained negative, we commenced maintenance
treatment with N-acetylcysteine at 2.5 g in 250 mL
5% dextrose solution infused over 4 hours twice daily
(100 mg/kg/day) from 26 June 2022 until viral
clearance on 18 July 2022. The patient was weaned
off inotropic agents and mechanical ventilation and
became dialysis and oxygen supplement independent.
He was discharged from the intensive care unit on
29 June 2022 and resumed immunosuppressive
therapy on 30 June 2022. He was discharged home
on 28 July 2022. The patient failed to develop any
antibody response against SARS-CoV-2 throughout
the infection (Fig).
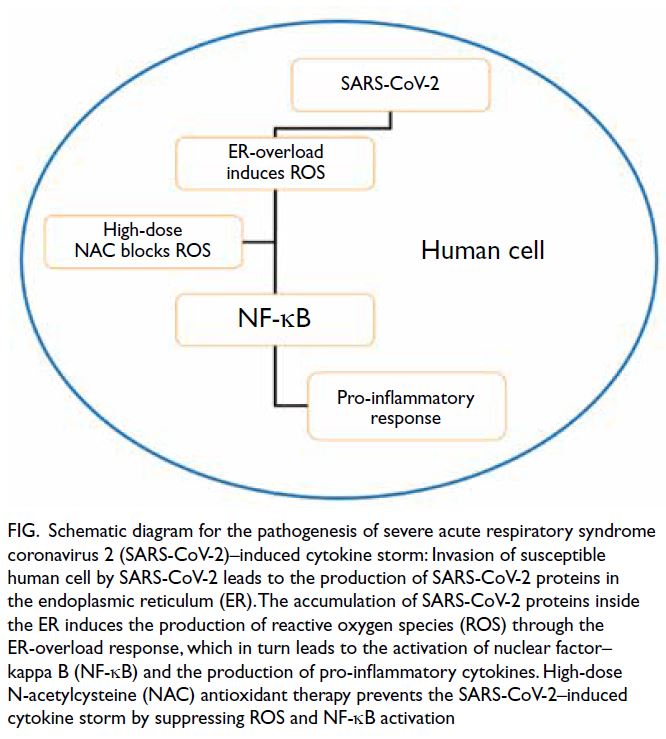
Figure. Schematic diagram for the pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–induced cytokine storm: Invasion of susceptible human cell by SARS-CoV-2 leads to the production of SARS-CoV-2 proteins in the endoplasmic reticulum (ER). The accumulation of SARS-CoV-2 proteins inside the ER induces the production of reactive oxygen species (ROS) through the ER-overload response, which in turn leads to the activation of nuclear factor–kappa B (NF-κB) and the production of pro-inflammatory cytokines. High-dose N-acetylcysteine (NAC) antioxidant therapy prevents the SARS-CoV-2–induced cytokine storm by suppressing ROS and NF-κB activation
Discussion
The SARS-CoV-2 mutates rapidly and relies on
host cell factors and physiological processes for its
entry, replication, and egress. These processes result
in cytopathic damage, cytokine dysregulation, and
death of host cells. These non-mutable key steps
inside the host may be novel targets for future
therapeutic strategies against these rapidly mutating
viruses. The endoplasmic reticulum (ER) stores
the majority of calcium ions and governs protein
translation. The accumulation of proteins in the ER
membrane, namely ER overload, leads to release of
calcium ions from the ER and production of reactive
oxygen species. This results in the activation of
nuclear factor–kappa B (NF-κB) and the release
of pro-inflammatory cytokines. The accumulation
of coronavirus spike protein in the ER membrane
results in an ER-overload response and cytokine
storm.3 Open reading frame 3a (ORF3a) protein,
ORF7a protein, membrane protein, and nucleocapsid
protein of SARS-CoV-2 are also NF-κB activators. Of
these four, ORF7a protein is the most potent NF-κB
inducer and pro-inflammatory cytokine producer.4
N-acetylcysteine is an antioxidant against reactive
oxygen species and is a potent NF-κB inhibitor.5 Oral
N-acetylcysteine administered at 600 mg thrice daily
has been shown to reduce mortality in hospitalised
SARS-CoV-2 patients.6 The clinical course of our patient suggests that high-dose N-acetylcysteine
antioxidant therapy was able to control the cytokine
storm of SARS-CoV-2 infection.
The SARS-CoV-2 virus mutates rapidly
to produce new variants that can evade human
antibody response and escape T-cell recognition
and clearance. New variants cause challenges to
the global effort in developing effective vaccines
and medications against SARS-CoV-2. Current
therapeutic strategies including vaccination, anti-viral
medications, and monoclonal antibodies
are directed against the mutable targets of SARS-CoV-2. The SARS-CoV-2 vaccines and monoclonal
antibodies that are highly effective against the
SARS-CoV-2 wild-type (Wuhan-Hu-1) strain failed to
confer adequate protection against the breakthrough
infection nor prevent antibody evasion of omicron
variants. Nonetheless high-dose N-acetylcysteine
therapy acts directly against the reactive oxygen
species and NF-κB activation in the ER-overload
response of the host, independent of viral mutation.
N-acetylcysteine has a complementary or even
synergistic role to therapeutic agents that act on the
mutable targets of SARS-CoV-2.
This case report illustrates that high-dose N-acetylcysteine
can protect against SARS-CoV-2–induced cytokine storm in an immunocompromised
host who could not elicit an antibody response.
By controlling the cytokine storm, this patient
coexisted with SARS-CoV-2 until viral clearance.
As of 16 October 2022, only 23.3% of people in low-income
countries had received at least one dose of
coronavirus disease 2019 vaccine. If the protection
afforded by high-dose N-acetylcysteine against
severe complications of SARS-CoV-2 infection in
patients without antibody response can be confirmed
in prospective studies, non-fully vaccinated people
and those with suboptimal antibody response
to vaccination may benefit. This may include
those with malignancies, on chemotherapy or
immunosuppressive medications, with inborn errors
of immunity, or with autoantibodies against type I
interferons who are prone to critical SARS-CoV-2
pneumonia. N-acetylcysteine is safe and a category
B drug for pregnancy. It is affordable for countries
with limited resources and has the potential to end
the coronavirus disease 2019 pandemic.
Author contributions
Concept or design: KY Lai.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: KY Lai, SY Au.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: KY Lai, SY Au.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have declared no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit
sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki and provided informed consent
for all treatments and procedures, and consent for publication.
References
1. Ungheri D, Pisani C, Sanson G, et al. Protective effect of
N-acetylcysteine in a model of influenza infection in mice. Int J Immunopathol Pharmacol 2000;13:123-8.
2. Lai KY, Ng WY, Chan PK, Wong KF, Cheng F. High-dose N-acetylcysteine therapy for novel H1N1 influenza
pneumonia. Ann Intern Med 2010;152:687-8. Crossref
3. Versteeg GA, van de Nes PS, Bredenbeek PJ, Spaan WJ. The
coronavirus spike protein induces endoplasmic reticulum
stress and upregulation of intracellular chemokine mRNA
concentrations. J Virol 2007;81:10981-90. Crossref
4. Su CM, Wang L, Yoo D. Activation of NF-κB and induction
of proinflammatory cytokine expressions mediated by
ORF7a protein of SARS-CoV-2. Sci Rep 2021;11:13464. Crossref
5. Hariharan A, Hakeem AR, Radhakrishnan S, Reddy MS,
Rela M. The role and therapeutic potential of NF-kappa-B pathway in severe COVID-19 patients. Inflammopharmacology 2021;29:91-100. Crossref
6. Izquierdo JL, Soriano JB, González Y, et al. Use of
N-acetylcysteine at high doses as an oral treatment
for patients hospitalized with COVID-19. Sci Prog
2022;105:368504221074574. Crossref

