Hong Kong Med J 2023 Dec;29(6):551–3 | Epub 8 Dec 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
The underestimated power of cooked meat in affecting plasma creatinine level: three case reports
CY So1; Toby CH Chan, MB, BS1; KY Yuet, BSc, FAACB1; Eugene YH Chan, FHKAM (Paediatrics)2; Terry TW Chow, FHKAM (Paediatrics)3; Matthew CW Yeung, MB, BS, FHKAM (Pathology)1; Alison LT Ma, FHKAM (Paediatrics), FRCPCH2; Frankie WT Cheng, FRCPCH, MD3; Chloe M Mak, PhD, MD1
1 Chemical Pathology Laboratory, Department of Pathology, Hong Kong Children’s Hospital, Hong Kong SAR, China
2 Division of Nephrology, Department of Paediatrics and Adolescent Medicine, Hong Kong Children’s Hospital, Hong Kong SAR, China
3 Division of Haematology and Oncology, Department of Paediatrics and Adolescent Medicine, Hong Kong Children’s Hospital, Hong Kong SAR, China
Corresponding author: Dr Chloe M Mak (makm@ha.org.hk)
Introduction
The use of plasma creatinine as biomarker for renal
function is not foolproof. Pre-analytical factors such
as dietary intake of cooked meat can significantly
influence plasma creatinine level, giving rise to
pseudo-renal failure. We report three cases of
paediatric oncology patients who presented with
spuriously high plasma creatinine level secondary
to ingestion of a large amount of cooked meat,
domestically prepared in the form of essence. The
frequent occurrence of such practice is likely rooted
in the traditional Chinese food culture of ingesting
meat essence as a tonic.
Case presentations
Case 1
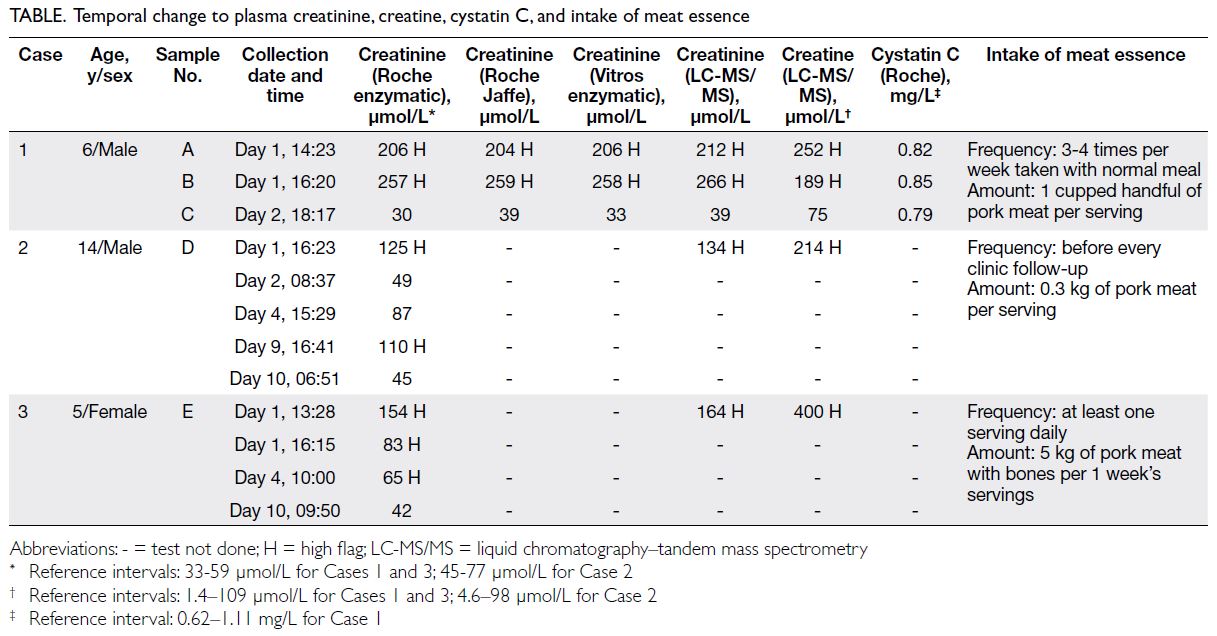
A drastic increase in plasma creatinine level to
206 μmol/L (reference interval [RI]: 33-59) from
normal baseline was noted in a 6-year-old boy with a
history of acute lymphocytic leukaemia in remission
during a routine pre-clinic blood test taken at 4 pm.
Urgent admission was arranged for suspected acute
kidney injury (AKI). On admission, his creatinine
level measured about 26 hours after the clinic visit
had spontaneously normalised in the absence of any
treatment. Urgent urinary system ultrasonography
and other blood tests were unremarkable. Clinically,
the patient was well and asymptomatic. He was
discharged uneventfully.
Case 2
Renal function test requested as part of pre-consolidation
chemotherapy assessment for a
14-year-old boy with B-cell acute lymphoblastic
leukaemia showed a rise of plasma creatinine level
to 125 μmol/L (RI: 45-77) from the normal baseline.
Intravenous fluid was started for suspected AKI
and the plasma creatinine level normalised the
following morning. However, a reassessment after 3 days showed an elevated creatinine level once again.
A third reassessment after 5 more days revealed a
creatinine level of 110 μmol/L. The patient was
admitted for intravenous rehydration with retesting
the following morning showing a normal plasma
creatinine level. Consolidation was eventually
started 9 days later than the initial planned date. The
patient remained well and asymptomatic throughout
the course.
Case 3
A sudden increase in creatinine level (154 μmol/L,
RI: 33-59) from the normal baseline was noted
during a pre-clinic blood test at 1 pm in a 5-year-old
girl with B-cell acute lymphoblastic leukaemia on
maintenance chemotherapy. Clinically, the patient
exhibited no symptoms. Subsequent blood tests at
4 pm and 3 days later showed gradual reduction
of creatinine levels to 83 μmol/L and 65 μmol/L,
respectively.
In light of this ‘outbreak’ of spuriously high
creatinine level in multiple paediatric oncology
patients, extensive investigations were performed
on the residual samples for suspected interference.
Analytical interference was excluded by dilution
study, and re-analysis performed on alternative
analyser platforms with the same enzymatic method
and by other methods, including Jaffe and liquid
chromatography–tandem mass spectrometry
(Table). A normal and stable plasma cystatin C level
was detected in Case 1, indicating that the actual
renal function remained stable despite the rise in
plasma creatinine level. Unfortunately, the residual
samples D and E for Cases 2 and 3, respectively,
were insufficient for cystatin C testing. The clinical
status and other renal function markers of both
cases had remained stable during the episode,
despite the spurious transient and abrupt increase in
creatinine levels. The levels also returned quickly to
normal without active management. Furthermore, the creatinine increase was found to be paired with
creatine increase in all three cases, up to 2 to 4 times
the upper limit of normal, indicating recent creatine
and creatinine loads.
Dietary history was pursued. Initially, all
parents denied excessive meat, fish, or egg intake,
but later disclosed habitual preparation of cooked
meat in the form of essence (燉肉汁) for their child.
Intriguingly, the caretakers had been frequently
preparing tonic by double-boiling a large amount
of pork meat in a slow cooker, a cooking method
resembling that of ‘chicken essence’, a popular
traditional health remedy in Asia, especially in
Chinese.
Discussion
Renal function can be conveniently estimated by
plasma creatinine level but it has its limitations that
should not be overlooked. The level can be influenced
by multiple patient factors such as age, sex, muscle
mass, tubular secretion, and dietary intake of cooked
meat.1
Foods rich in creatine include meat, fish, and
poultry. Red meat and fish contain 4 to 10 g creatine
per kilogram.2 The average daily creatine intake is
estimated to be 0.54 to 0.60 g in children and 0.81
to 0.87 g in adults.2 Cooking enhances the in vitro
conversion of creatine in meat to creatinine that
is readily absorbed in the gastrointestinal tract.
Experiments showed that a single cooked meat meal (225 g boiled beef) can lead to a sharp and
transient increase in plasma creatinine, with a peak
postprandial increase in adults of 52%, followed by a
gradual decrease to baseline after 12 to 24 hours.3 4
This phenomenon, also known as ‘goulash effect’, may
affect clinical interpretation of plasma creatinine
level and the estimated glomerular filtration rate.5 6 7
The three paediatric cases described above
demonstrated a transient exaggerated increase in
creatinine following consumption of homemade
meat essence. The rise in plasma creatinine level
was 7.5-fold in Case 1 and mimicked a stage 3 AKI.
Clinical features including normal urine output
and stable haemodynamics hinted at an inaccurate
estimate of renal function by plasma creatinine level.
An alternative blood test that is less susceptible
to interference such as cystatin C would provide
comforting reassurance as illustrated in Case 1.
Locally, Lee et al8 reported a case of pseudo-renal
failure (creatinine level of 222 μmol/L) in a healthy
14-month-old boy secondary to consumption of
domestically prepared concentrated meat broth.
Aggarwal et al9 reported a case of fluctuating
plasma creatinine level in a transplant recipient
who consumed homemade meat soup before blood
taking, hindering optimal clinical management.
In this series, the caretakers had been preparing
meat essence as tonics for their sick child, a healthy
remedy favoured among Chinese parents. It is worth
noting that this important piece of history might be missed if medical professionals do not question
parents about their child’s diet. Pseudo-renal failure
secondary to consumption of homemade meat
essence resulted in unnecessary hospital admissions,
blood taking and imaging studies, as well as a delay
in scheduled chemotherapy treatment.
This series highlights domestic preparation of
meat essence as a recurring cause of pseudo-renal
failure in the local population. Medical professionals
should be alert to the influence of cooked meats
on plasma creatinine level. Early recognition can
prevent excessive or unnecessary treatment and
investigations. An alternative blood test for renal
function, eg, cystatin C, should be considered in the
presence of a spurious rise in plasma creatinine level.
Serum cystatin C test is recently available in our
laboratory in the Hong Kong Children’s Hospital.
Parental advice to avoid excessive cooked meat
intake prior to blood taking will also reduce future
occurrence.
Author contributions
All authors contributed to the concept or design, acquisition
of data, analysis or interpretation of data, drafting of the
manuscript, and critical revision of the manuscript for
important intellectual content. All authors had full access to
the data, contributed to the study, approved the final version
for publication, and take responsibility for its accuracy and
integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The study was approved by the Hong Kong Children’s Hospital Research Ethics Committee (Ref No.: HKCH-REC-2021-059). The requirement for patient consent was waived by the
Committee due to the retrospective nature of the study and
the use of anonymised data.
References
1. Samra M, Abcar AC. False estimates of elevated creatinine.
Perm J 2012;16:51-2. Crossref
2. Korovljev D, Todorovic N, Stajer V, Ostojic SM. Temporal
trends in dietary creatine intake from 1999 to 2018: an
ecological study with 89,161 participants. J Int Soc Sports
Nutr 2021;18:53. Crossref
3. Mayersohn M, Conrad KA, Achari R. The influence of a
cooked meat meal on creatinine plasma concentration and
creatinine clearance. Br J Clin Pharmacol 1983;15:227-30. Crossref
4. Jacobsen FK, Christensen CK, Mogensen CE, Andreasen F,
Heilskov NS. Pronounced increase in serum creatinine
concentration after eating cooked meat. Br Med J
1979;1:1049-50. Crossref
5. Nair S, O’Brien SV, Hayden K, et al. Effect of a cooked
meat meal on serum creatinine and estimated glomerular
filtration rate in diabetes-related kidney disease. Diabetes
Care 2014;37:483-7. Crossref
6. Pimenta E, Jensen M, Jung D, Schaumann F, Boxnick S,
Truebel H. Effect of diet on serum creatinine in healthy
subjects during a phase I study. J Clin Med Res 2016;8:836-9. Crossref
7. Cottrell A. Renal function. In: Probert JL, editor. Urology:
an atlas of investigation and diagnosis. Oxford: Clinical
Publishing; 2009.
8. Lee HC, Mak MC, Tong RC, et al. Congee: a cause of gross
but transient elevation in plasma creatinine concentration.
Br J Biomed Sci 2011;68:47-8. Crossref
9. Aggarwal S, Sukkar L, Wynter L, Richards K, Cheung J, Chadban SJ. Dangers of broth after transplantation. Nephrology (Carlton) 2015;20:297-9. Crossref