Hong Kong Med J 2023 Dec;29(6):532–41 | Epub 30 Jun 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE CME
Consensus recommendations for the screening, diagnosis, and management of Helicobacter pylori infection in Hong Kong
WK Leung, MD, FHKAM (Medicine)1,2; KS Cheung, MD, MPH1; Philip CO Sham, MB, BS (HK), FHKAM (Paediatrics)3; Raymond SY Tang, MD4; CK Loo, FHKAM (Medicine), FRCP (London)5; Axel SJ Hsu, MB, BS (HK), FHKCP2,6; TK Cheung, PhD, FRACP5; LY Lam, MB, BS (HKU)7; Billy CF Chiu, FHKAM (Family Medicine), MPH (HK)3; Justin CY Wu, MB, ChB, MD2,4
1 Department of Medicine, Queen Mary Hospital/The University of Hong Kong, Hong Kong SAR, China
2 The Hong Kong Society of Gastroenterology, Hong Kong SAR, China
3 Gleneagles Hong Kong Hospital, Hong Kong SAR, China
4 Department of Medicine and Therapeutics, Prince of Wales Hospital/The Chinese University of Hong Kong, Hong Kong SAR, China
5 Private Practice, Hong Kong SAR, China
6 Hong Kong Sanatorium & Hospital, Hong Kong SAR, China
7 St Teresa’s Hospital, Hong Kong SAR, China
Corresponding author: Prof WK Leung (waikleung@hku.hk)
Abstract
Helicobacter pylori infection causes chronic
gastric inflammation that contributes to various
gastroduodenal diseases, including peptic ulcer and
gastric cancer. Despite broad regional variations,
the prevalence of resistance to antibiotics used to
manage H pylori infection is increasing worldwide;
this trend could hinder the success of eradication
therapy. To increase awareness of H pylori and
improve the diagnosis and treatment of its infection
in Hong Kong, our consensus panel proposed a set
of guidance statements for disease management.
We conducted a comprehensive review of literature
published during 2011 and 2021, with a focus
on articles from Hong Kong or other regions of
China. We evaluated the evidence using the Oxford
Centre for Evidence-Based Medicine’s 2011 Levels
of Evidence and the Grading of Recommendations
Assessment, Development and Evaluation (GRADE)
system and sought consensus through online voting
and a subsequent face-to-face meeting, which
enabled us to develop and refine the guidance
statements. This report consists of 24 statements
regarding the epidemiology and burden, screening
and diagnosis, and treatment of H pylori. Key
guidance statements include a recommendation
to use the test-and-treat approach for high-risk individuals, as well as the confirmation that triple
therapy with a proton pump inhibitor, amoxicillin,
and clarithromycin remains a valid first-line option
for adults and children in Hong Kong.
Introduction
Antibiotics are the primary treatment for
Helicobacter pylori; however, resistance to common
antibiotics used in eradication therapy (eg,
clarithromycin, metronidazole, and quinolones)
is increasing worldwide, thereby reducing the
expected therapeutic benefit.1 Thus, there is an
urgent need for an updated management guide that
considers susceptibility patterns, disease prevalence,
and patient factors in Hong Kong. Accordingly, a
panel of 10 experts from Hong Kong gathered to
review recently published evidence regarding the
management of H pylori infection to develop this
consensus report.
PubMed was searched for published peer-reviewed
articles in English on the epidemiology,
screening, diagnosis, and treatment of H pylori
infection, with a focus on Hong Kong and China. The search included clinical trials (randomised controlled
trials [RCTs] and controlled clinical trials), practice
guidelines, meta-analyses, systematic reviews, and
observational studies from January 2011 to August
2021.
In September 2021, the panel assigned
consensus topics to specific members for literature
review and statement drafting, followed by a
discussion in October 2021. The Oxford Centre for
Evidence-Based Medicine’s 2011 Levels of Evidence2
and the GRADE (Grading of Recommendations
Assessment, Development and Evaluation) system
were used to evaluate level of evidence and classify
recommendations, respectively. Details of GRADE
classification are shown in the online supplementary Table.
All participants were asked to indicate their
level of agreement using a Likert scale (1: completely agree; 2: agree with some reservations; 3: agree with
major reservations; 4: disagree with reservations;
5: completely disagree). Statements were modified
as necessary, and voting was repeated online in
November 2021. Consensus was achieved if at least
75% of the panel members agreed with a statement
(completely or with reservations). Statements
regarding consensus recommendations for the
screening, diagnosis, and management of H pylori
infection in Hong Kong are detailed below.
Epidemiology and burden
Statement 1: Although the prevalence of H pylori infection in many developed countries has declined in
recent decades, epidemiological data for Hong Kong,
except in children, are limited. (quality of evidence:
2/3; strength of recommendation: not applicable;
level of consensus: 100%)
Global and regional estimates published
in 2017 revealed that the prevalences of H pylori
infection were 55.8% in China and 53.9% in Taiwan.3
No prevalence data for Hong Kong have been
reported since 2011. Although the prevalences in
many countries in Europe and Northern America
have declined since 2000, the prevalences in Asia
before and after 2000 were similar (53.6% vs 54.3%).3
Two retrospective studies and a population-based
study explored the H pylori infection rate in Hong
Kong children. In 2008, the estimated rate of H
pylori infection in healthy school children (n=2480)
was 13.1%.4 Among 602 children who underwent
esophagogastroduodenoscopy at a tertiary centre for
peptic ulcer symptoms, the H pylori infection rate
decreased from 25.6% in 2005 to 12.8% in 2017.5 6
Statement 2: Although the rate of H pylori reinfection
remains low (<2%) in the Chinese population, it
may be higher in children than in adults. (quality of
evidence: 3; grade of recommendation: not applicable;
level of consensus: 80%)
In a systematic review of 132 studies, the global
annual rates of H pylori recurrence, reinfection,
and recrudescence were 4.3%, 3.1%, and 2.2%,
respectively. The global rates of H pylori recurrence
generally remained stable in the 1990s, 2000s, and
2010s, but data varied according to region.7
Data regarding the rates of H pylori recurrence, reinfection, and recrudescence in Hong Kong adults
are limited. A community-based study showed that
the rate of H pylori reinfection in Taiwan was 0.34 to
0.95 per 100 person-years between 2008 and 2018.8
In 2020, a prospective cohort study in China showed
that the annual rate of H pylori reinfection was 1.5%
per person-year.9
The rates of H pylori reinfection may be higher in children. In a study from Baoding in Hubei, China,
the recurrence rate was 18.8% (41/218 children with
successful follow-up).10 Moreover, the rate was
higher in children aged ≤10 years than in children
aged >10 years (22.8% vs 7.1%, P=0.01). Similarly, a
Bolivian population-based study showed a higher
annual recurrence rate in younger children than in
older children: 20% for children aged <5 years, 20%
for children aged 5 to 9 years, 8% among children
aged 10 to 14 years, and 8% among individuals aged
≥15 years.11
Statement 3: Helicobacter pylori infection in adults
has been associated with increased risks of gastric
adenocarcinoma, peptic ulcer disease, non-ulcer
dyspepsia, and mucosa-associated lymphoid tissue
(MALT) lymphoma. Eradication of H pylori has been
shown to reduce gastric cancer incidence, reduce peptic
ulcer recurrence, and provide symptomatic relief in
H pylori–positive patients with non-ulcer dyspepsia.
(quality of evidence: 1; grade of recommendation: not
applicable; level of consensus: 90%)
Helicobacter pylori infection is considered
an important causal risk factor for non-cardia
gastric adenocarcinoma.12 The estimated global
burden of gastric cancer attributable to H pylori is
89%.13 The odds ratio of gastric cancer onset among
patients with H pylori infection ranges from 5.9 to
34.5.14 15 Usually, a high incidence of gastric cancer
is associated with a high prevalence of H pylori
infection.12 16
A reduced risk of gastric cancer after H pylori
eradication has been demonstrated in interventional
trials, including RCTs.17 18 19 20 21 22 23 To prevent one case of
gastric cancer in H pylori–positive patients from a
region with a high risk of gastric cancer (eg, China),
the minimum number needed to treat was 15
according to a meta-analysis of six RCTs.24 In 2018, a territory-wide study of 73 237 H pylori–infected
patients in Hong Kong showed that eradication was
associated with a reduced risk of gastric cancer,
particularly among patients aged ≤60 years.25 A meta-analysis
of 24 studies also showed that the benefit
of H pylori eradication for gastric cancer protection
was greater in patients with endoscopically resected
early gastric cancer compared with asymptomatic
patients; moreover, eradication was associated with
a reduced incidence of metachronous recurrence.26
The available evidence suggests that, even when H
pylori treatment is initiated after the development of
atrophic gastritis and metaplasia, the risk of gastric
cancer is reduced.
Helicobacter pylori is a causal risk factor
for peptic ulcer disease; its eradication therapy is
effective in treating and preventing the recurrence of
both gastric and duodenal ulcers.27 28
There is a potential causal link between H pylori infection and dyspeptic symptoms. Helicobacter
pylori eradication had a small but statistically
significant effect on the relief of dyspeptic symptoms
in H pylori–positive patients.29
Gastric MALT lymphoma was also associated
with H pylori infection; remission was achieved in
77.8% of patients after successful eradication.30
Screening and diagnosis
Statement 4: Considering the declining incidence of
gastric cancer in Hong Kong, screening for H pylori in
the general population is not recommended. (quality
of evidence: 1; grade of recommendation: conditional;
level of consensus: 90%)
A screen-and-treat strategy for H pylori is most cost-effective in regions with high gastric cancer
incidence (ie, 20 per 100 000 person-years).26 The
2020 age-standardised incidence of gastric cancer in
Hong Kong was 8.7 and 5.3 per 100 000 person-years
in male and female, respectively.31 Because of this
declining incidence, a screen-and-treat strategy may
not be cost-effective for gastric cancer prevention in
Hong Kong.
Statement 5: Among adults without gastric
symptoms, individuals at high risk of gastric cancer
(eg, individuals with a family history of gastric cancer)
should be tested and (if they test positive) treated for
H pylori. Otherwise, routine testing of asymptomatic
household members or family members of H pylori–infected adults is not recommended. (quality of
evidence: 1; grade of recommendation: strong; level
of consensus: 90%)
Statement 6: Adults with non-ulcer dyspepsia, peptic
ulcer disease, and early gastric cancer after endoscopic
treatment should be tested and (if they test positive)
treated for H pylori. (quality of evidence: 1; grade of
recommendation: strong; level of consensus: 100%)
Statement 7: Adults with gastric biopsy results
showing atrophy, intestinal metaplasia, or dysplasia
should be tested and (if they test positive) treated
for H pylori. (quality of evidence: 1; grade of
recommendation: strong; level of consensus: 100%)
Family history, atrophic gastritis, and intestinal
metaplasia are established risk factors for gastric
cancer.32 33 Therefore, it is prudent to test for and
treat H pylori in patients with a family history or pre-cancerous
gastric lesions.
Statement 8: Adults planning to begin long-term low-dose aspirin treatment should be tested and (if
they test positive) treated for H pylori. (quality of
evidence: 3; grade of recommendation: conditional;
level of consensus: 90%)
Statement 9: Adult patients planning to begin other
non-aspirin non-steroidal anti-inflammatory drugs,
antiplatelets, and anticoagulants should be tested
and (if they test positive) treated for H pylori. (quality
of evidence: 3; grade of recommendation: conditional;
level of consensus: 70%)
Low-dose aspirin, non-steroidal anti-inflammatory
drugs, anticoagulants, and
antiplatelets can increase the risk of gastrointestinal
(GI) bleeding.34 35 There is limited and conflicting
evidence regarding the interaction among these
agents, H pylori, and GI bleeding.36 37 38 39 Therefore,
the benefit of testing and treatment for all users
of these agents is unclear. However, the treatment
of H pylori infection along with the use of
gastroprotective strategies could mitigate the risk
of GI complications, particularly in patients at high
risk of GI bleeding.32 40 41 Thus, despite the conflicting
evidence, the consensus panel also favoured testing
and treatment for H pylori infection in these
patients.
Statement 10: Adults with unexplained iron
deficiency anaemia, vitamin B12 deficiency, or
immune thrombocytopenic purpura should be
tested and (if they test positive) treated for H pylori.
(quality of evidence: 1/2; grade of recommendation:
conditional; level of consensus: 90%)
Iron deficiency anaemia was associated with
H pylori infection in both adults and children. The
effect of iron therapy for iron deficiency anaemia
may be enhanced with H pylori treatment.42 43 44 45 46 In
recent decades, systematic reviews have shown that
H pylori eradication can also improve platelet counts
in adult and paediatric patients with idiopathic
thrombocytopenic purpura.47 48 49
However, this panel does not recommend
testing and treatment for all children with chronic
idiopathic thrombocytopenic purpura. Additionally,
the identification of iron deficiency anaemia
aetiology in children should be prioritised over the
detection and treatment of H pylori.
Statement 11: Routine H pylori testing in asymptomatic children is not recommended.
However, children with peptic ulcer disease should be
tested and (if they test positive) treated for H pylori.
(quality of evidence: 2/3; grade of recommendation:
strong; level of consensus: 90%)
Helicobacter pylori infection in children
is mainly asymptomatic and rarely causes
complications; thus, routine non-invasive testing
in an otherwise asymptomatic child is not usually
recommended. When a child presents with GI
symptoms, the clinical investigation should focus on
identifying the cause of the child’s symptoms, rather
than solely confirming the presence of H pylori.50 51
Statement 12: Non-invasive tests, including the urea
breath test and (preferably monoclonal) stool antigen
test, are highly accurate for the initial diagnosis and
follow-up of H pylori. (quality of evidence: 2; grade of
recommendation: not applicable; level of consensus:
90%)
The carbon-13 urea breath test and stool
antigen test are non-invasive diagnostic tests with
high accuracy in the detection of H pylori. The
carbon-13 urea breath test has a sensitivity of 95%
to 98% and a specificity of 90% to 97%.52 53 The
monoclonal stool antigen test has a sensitivity of
90% to 98% and a specificity of 90% to 97%.52 54
For post-eradication therapy follow-up,
reliable results can be obtained at 2 weeks after
discontinuation of proton pump inhibitors (PPIs) and
at least 4 weeks after discontinuation of antibiotics
and bismuth.32
Statement 13: Serological testing is not recommended
for initial diagnosis and post-eradication follow-up
of H pylori. (quality of evidence: 2; grade of
recommendation: conditional; level of consensus: 100%)
Serological testing has low accuracy and
high false-negative rates for initial diagnosis32; it is
not recommended for post-eradication follow-up
because it can detect antibodies from past infections.55
However, it may be useful in the management of
some clinical conditions characterised by decreased
bacterial load (eg, GI bleeding, atrophic gastritis,
gastric MALT lymphoma, and gastric cancer); other
tests can lose sensitivity for these conditions.32
Statement 14: For all patients who undergo endoscopy,
the initial diagnosis of H pylori can be made by the
following methods: rapid urease test, histology with
or without specific staining, and culture. (quality of
evidence: 2; grade of recommendation: strong; level of
consensus: 100%)
Gastric biopsies are ideal specimens for
diagnostic rapid urease tests or histopathological
assessments.32 Samples generally should be collected
from both the antrum and corpus. Rapid urease tests can be used for quick assessment, but specimens
with low bacterial loads can yield false-negative
results.32 56 Culture-based detection of H pylori has
comparatively low sensitivity and is usually reserved
for instances where antimicrobial susceptibility
testing is needed.
Additional information about screening for
H pylori in pregnancy and diagnosis for H pylori
in children are shown in the online supplementary Appendix.
Treatment
Statement 15: The choice of H pylori eradication therapy should be based on H pylori microbial
resistance patterns and antibiotic stewardship in
Hong Kong, as well as the efficacy of gastric acid
suppression. The regimen should be simple to use
and well-tolerated, with good compliance and high
efficacy (>85%). (quality of evidence: 1; grade of
recommendation: strong; level of consensus: 100%)
In addition to tolerability and compliance,
key H pylori treatment considerations include its
susceptibility and resistance to antimicrobials, both
of which demonstrate temporal and geographical
variability.32 40 57 58
The degree of gastric acid suppression is one of
the most important factors in determining the success
of H pylori eradication.2 59 The dose, frequency,
and potency of PPIs, as well as host genetics
(hepatic cytochrome P450 2C19 polymorphism),
can influence gastric pH. The most effective acid
suppression regimen should be used to increase
antibiotic bioavailability.60 Analyses of potassium-competitive
acid blockers have shown that greater
acid suppression can improve eradication success.61
A longer eradication therapy interval could also
improve the eradication rate.
Statement 16: In the first-line setting for H pylori
eradication, possible therapies include (a) triple
therapy with a PPI, clarithromycin, and amoxicillin
for 14 days; and (b) bismuth quadruple therapy with
a PPI, tetracycline, metronidazole, and a bismuth
salt for 10 to 14 days. (quality of evidence: 1/2; grade
of recommendation: strong/conditional; level of
consensus: 100%)
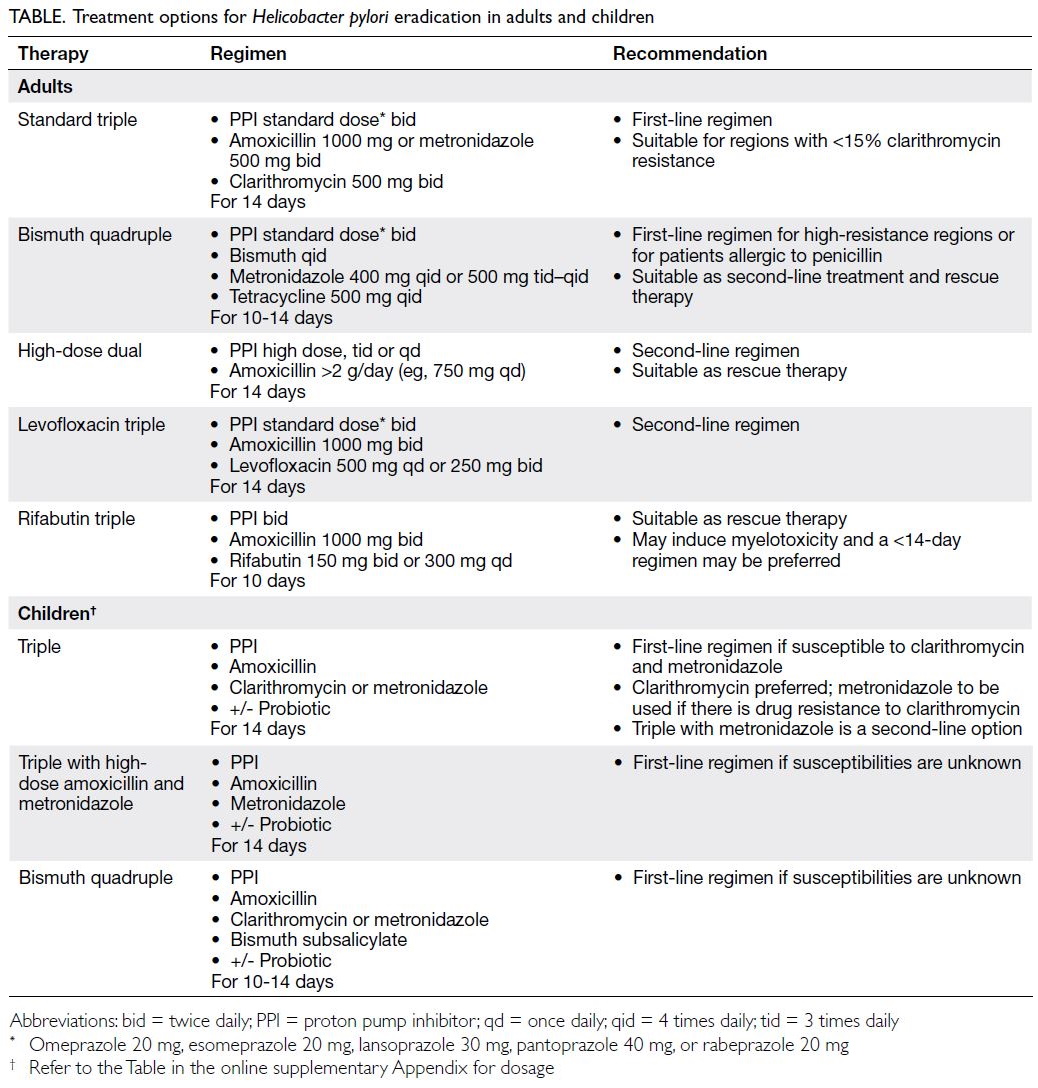
Triple therapy with a PPI, clarithromycin, and
amoxicillin (Table) remains the first-line option in
regions with clarithromycin resistance <15% and a
local eradication rate of ≥85%.32 57 Patients allergic
to amoxicillin should receive metronidazole. If
clarithromycin resistance exceeds 15%, bismuth
quadruple therapy is recommended as another first-line
option (ie, a PPI, tetracycline, metronidazole,
and a bismuth salt). According to a meta-analysis
published in 2018, the prevalence of resistance
to clarithromycin was 10% (95% confidence
interval=5%-17%) in Hong Kong; the prevalence of resistance to metronidazole was 53% (95%
confidence interval=39%-66%).62 A more recent
population-based study in Hong Kong showed that
the overall failure rate of clarithromycin-based triple
therapy was 10.1% during the period from 2003 to
2018.63 Compared with the 7-day regimen, a 14-day regimen of triple therapy is usually recommended
because it produces better eradication rates.57 64
Randomised trials have demonstrated
eradication rates of >92% (intent-to-treat analysis)
when bismuth quadruple therapy is used as empirical
first-line treatment.65 66 Studies from Taiwan and
Texas of the United States revealed that treatment
intervals of 10 to 14 days led to eradication rates of
>90%.65 67 However, the tolerability and availability of bismuth compounds could limit the widespread use
of bismuth-based therapy.65
Statement 17: In the second-line setting for H pylori
eradication (ie, after unsuccessful clarithromycin-based
triple therapy), possible therapies include (a)
bismuth quadruple therapy with a PPI, tetracycline,
metronidazole, and a bismuth salt for 10 to 14 days;
(b) high-dose PPI–amoxicillin dual therapy for 14
days; and (c) levofloxacin-containing triple therapy
with a PPI and amoxicillin for 14 days. (quality of
evidence: 1/2; grade of recommendation: conditional;
level of consensus: 100%)
Second-line treatment should not repeat the
previous regimen. The reuse of antibiotics that were previously unsuccessful (eg, clarithromycin and
levofloxacin, both of which commonly cause post-exposure
resistance) should be avoided. However, as amoxicillin and tetracycline have low rates of resistance, they can be reused. Metronidazole can
also be reused if administered in combination with
bismuth salt.57
If testing is feasible, the choice of therapy should
be guided by antimicrobial susceptibility testing and
administered with the optimal treatment interval.32
Bismuth quadruple therapy can be regarded
as second-line treatment when antimicrobial
susceptibility testing is unavailable.32 High-dose
dual therapy (ie, high-dose PPI and amoxicillin) is
emerging as a second-line treatment because of its
favourable eradication rates.68 69 Levofloxacin-based
triple therapy with amoxicillin and a PPI may be
considered if bismuth-based therapy was used as
first-line treatment.70 71 72 73 However, a recent report showed that the prevalence of levofloxacin resistance
in Hong Kong was 17%.63
Quinolones and antibiotics in the tetracycline
class are not currently licensed for use in young
children, further limiting second-line treatment
options. However, the inclusion of levofloxacin or
tetracycline in triple therapy may be considered for
adolescents.50
Statement 18: After unsuccessful second-line
treatment, rescue therapies include (a) bismuth
quadruple therapy with a PPI, tetracycline,
metronidazole, and a bismuth salt for 10 to 14 days;
(b) high-dose PPI–amoxicillin dual therapy for 14
days; and (c) rifabutin-containing therapy with a
PPI and amoxicillin for 10 days. (quality of evidence:
2; grade of recommendation: conditional; level of
consensus: 100%)
Similar to the approach involved in selection of
second-line treatment, previously unused regimens
may be regarded as rescue therapy. Regions with
high fluoroquinolone resistance may consider a
rifabutin-containing regimen (usually with a PPI
and amoxicillin).32 40 57 Rifabutin use should be
limited because of its potential for myelotoxicity; a
10-day regimen of rifabutin (300 mg/day) is usually
recommended.74 75 76 Another concern regarding the
use of rifabutin is the potential for acquired rifamycin
resistance, particularly in regions where tuberculosis
is endemic.
Statement 19: The use of probiotics as adjunctive
therapy to reduce the side-effects associated with H
pylori eradication therapy should be individualised.
(quality of evidence: 1; grade of recommendation:
qualified; level of consensus: 90%)
Probiotics (eg, Lactobacilli) may help to
ameliorate treatment-related side-effects such
as diarrhoea.32 40 57 Eradication rates may also be improved when probiotics are administered before and after H pylori treatment, for an interval of >2 weeks, or in combination with bismuth quadruple therapy.77
Statement 20: Antibiotic susceptibility testing can be
considered after at least two empirical therapies with
different antimicrobial agents have been unsuccessful.
(quality of evidence: 1; grade of recommendation:
conditional; level of consensus: 90%)
A recent meta-analysis showed that
antimicrobial susceptibility–guided therapy was
slightly more effective than empirical therapy.78 The
available evidence suggests that an understanding
of the antimicrobial susceptibility profile can guide
antimicrobial selection and improve eradication,
particularly in patients for whom multiple therapies
have been unsuccessful.
Statement 21: There are insufficient data to
provide solid recommendations concerning medical
treatment for H pylori infection in children. The
optimal age for eradication therapy in children also
requires further investigation. (quality of evidence:
2/3; grade of recommendation: conditional; level of
consensus: 100%)
The treatment of H pylori in children is not
usually recommended. There are a few indications
for which treatment should be carefully considered:
incidental findings during endoscopy, findings of
ulceration or erosion, refractory iron deficiency
anaemia, and chronic idiopathic thrombocytopenic
purpura.50
Statement 22: H pylori eradication may worsen
gastroesophageal reflux disease in some patients.
(quality of evidence: 3; grade of recommendation: not
applicable; level of consensus: 90%)
In a meta-analysis, the pooled results of five
cohort studies suggested that there is an increased
risk of erosive gastroesophageal reflux disease in
patients with peptic ulcer disease who are undergoing
eradication therapy; however, this risk was not
supported by the pooled results of seven RCTs in
the same meta-analysis.79 In the past decade, meta-analyses
also revealed that eradication therapy was
not significantly associated with the development
of gastroesophageal reflux disease.80 81 Generally,
H pylori treatment does not have a clinically
significant effect on acid production.
Statement 23: Patients may gain weight after H pylori eradication; therefore, lifestyle advice should be offered as needed. (quality of evidence: 2; grade of
recommendation: qualified; level of consensus: 90%)
A meta-analysis showed that H pylori
eradication increased body weight and body mass
index, but it did not influence insulin resistance, fasting blood glucose, or lipid parameters.82 The
mechanisms that underlie weight gain after H
pylori eradication may be multifactorial, including
increased appetite related to changes in ghrelin
level, the resolution of dyspepsia and changes in gut
microbiota.83 84 85 86 Weight monitoring is advisable after
eradication therapy.
Statement 24: All patients should be tested for H pylori after eradication therapy. (quality of evidence: not applicable; grade of recommendation: strong;
level of consensus: 100%)
From a practical perspective, the confirmation
of eradication therapy success is strongly
recommended, particularly because persistent
H pylori infection can lead to complications.32 40
Considering the increasing prevalence of antibiotic
resistance, there is an emerging clinical need to
confirm H pylori clearance after eradication.
The urea breath test, stool antigen test, and
endoscopy-based assessments (eg, rapid urease test
and histology) have comparatively high sensitivity
and specificity for H pylori; these approaches may
be selected according to availability and patient
circumstances. Non-endoscopic tests should be
performed at least 4 weeks after eradication therapy
and/or 2 weeks after PPI treatment.32 40
Additional information regarding treatment
for H pylori in children is shown in the online supplementary Appendix.
Conclusion
After thorough review of the most recent evidence,
the consensus panel highlighted the importance of
appropriate diagnosis and treatment for patients
with H pylori infection to prevent complications.
Our current recommendations may differ from other
regions; in particular, standard triple therapy remains
a first-line option because clarithromycin resistance
is still relatively low in Hong Kong. Moreover, our
recommendations may preclude unnecessary testing
(particularly in asymptomatic children), facilitate
rational use of antibiotics, and improve eradication
rates and clinical outcomes.
Author contributions
Development of clinical questions: WK Leung, JCY Wu.
Retrieval of evidence: All authors.
Analysis or interpretation of evidence: All authors.
Discussion and finalisation of evidence and statements: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: WK Leung, JCY Wu.
Retrieval of evidence: All authors.
Analysis or interpretation of evidence: All authors.
Discussion and finalisation of evidence and statements: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: WK Leung, JCY Wu.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
WK Leung has participated in advisory boards for Roche
Diagnostics and Harbour BioMed. KS Cheung has received
research grants from the Hong Kong SAR Government,
consultant fees from the Xela Group, honoraria from
Janssen Pharmaceuticals, meeting support from Takeda
Pharmaceutical Company, and has participated in advisory
boards for Janssen Pharmaceuticals and AstraZeneca. RSY
Tang has received support from AstraZeneca for laboratory
test kits.
Acknowledgement
The authors thank MIMS Hong Kong for meeting logistics support and medical writing support, funded by AstraZeneca.
AstraZeneca had no role in study design, data collection/analysis/interpretation, or manuscript preparation.
References
1. Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health
Organization regions. Gastroenterology 2018;155:1372-82.e17. Crossref
2. Malfertheiner P, Link A, Selgrad M. Helicobacter pylori: perspectives and time trends. Nat Rev Gastroenterol Hepatol 2014;11:628-38. Crossref
3. Hooi JK, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 2017;153:420-9. Crossref
4. Tam YH, Yeung CK, Lee KH, et al. A population-based study of Helicobacter pylori infection in Chinese children resident in Hong Kong: prevalence and potential risk
factors. Helicobacter 2008;13:219-24. Crossref
5. Wong KK, Chung PH, Lan LC, Lin SC, Tam PK. Trends in the prevalence of Helicobacter pylori in symptomatic
children in the era of eradication. J Pediatr Surg
2005;40:1844-7. Crossref
6. Tang MY, Chung PH, Chan HY, Tam PK, Wong KK. Recent trends in the prevalence of Helicobacter pylori in
symptomatic children: a 12-year retrospective study in a
tertiary centre. J Pediatr Surg 2019;54:255-7. Crossref
7. Hu Y, Wan JH, Li XY, Zhu Y, Graham DY, Lu NH. Systematic review with meta-analysis: the global recurrence rate of
Helicobacter pylori. Aliment Pharmacol Ther 2017;46:773-9.Crossref
8. Chiang TH, Chang WJ, Chen SL, et al. Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: a long-term cohort study on Matsu Islands. Gut
2021;70:243-50. Crossref
9. Xie Y, Song C, Cheng H, et al. Long-term follow-up of Helicobacter pylori reinfection and its risk factors after initial eradication: a large-scale multicentre, prospective
open cohort, observational study. Emerg Microbes Infect
2020;9:548-57. Crossref
10. Zhang Y, Dong Q, Tian L, et al. Risk factors for recurrence of Helicobacter pylori infection after successful eradication in Chinese children: a prospective, nested case-control study. Helicobacter 2020;25:e12749. Crossref
11. Sivapalasingam S, Rajasingham A, Macy JT, et al. Recurrence of Helicobacter pylori infection in Bolivian
children and adults after a population-based “screen and
treat” strategy. Helicobacter 2014;19:343-8. Crossref
12. Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J Gastroenterol Hepatol 2010;25:479-86. Crossref
13. Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer 2015;136:487-90. Crossref
14. Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001;345:784-9. Crossref
15. Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts.
Gut 2001;49:347-53. Crossref
16. Sipponen P, Kimura K. Intestinal metaplasia, atrophic gastritis and stomach cancer: trends over time. Eur J
Gastroenterol Hepatol 1994;6 Suppl 1:S79-83.
17. Wong BC, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 2004;291:187-94. Crossref
18. Zhou L, Lin S, Ding S, et al. Relationship of Helicobacter pylori eradication with gastric cancer and gastric mucosal histological changes: a 10-year follow-up study. Chin Med J (Engl) 2014;127:1454-8.
19. Leung WK, Lin SR, Ching JY, et al. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut
2004;53:1244-9. Crossref
20. Saito D, Boku N, Fujioka T, et al. Impact of H. pylori eradication on gastric cancer prevention: endoscopic results of the Japanese Intervention Trial (JITHP-Study).
A randomized multi-center trial. Gastroenterology
2005;128(Supp 2):A4.Abstract 23.
21. Li WQ, Zhang JY, Ma JL, et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a
randomized intervention trial. BMJ 2019;366:l5016. Crossref
22. Wong BC, Zhang L, Ma JL, et al. Effects of selective
COX-2 inhibitor and Helicobacter pylori eradication on
precancerous gastric lesions. Gut 2012;61:812-8. Crossref
23. Choi IJ, Kim CG, Lee JY, et al. Family history of gastric cancer and Helicobacter pylori treatment. N Engl J Med 2020;382:427-36. Crossref
24. Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut 2020;69:2113-21. Crossref
25. Leung WK, Wong IO, Cheung KS, et al. Effects of
Helicobacter pylori treatment on incidence of gastric
cancer in older individuals. Gastroenterology 2018;155:67-75. Crossref
26. Lee YC, Chiang TH, Chou CK, et al. Association between Helicobacter pylori eradication and gastric
cancer incidence: a systematic review and meta-analysis.
Gastroenterology 2016;150:1113-24.e5. Crossref
27. Lanas A, Chan FK. Peptic ulcer disease. Lancet 2017;390:613-24. Crossref
28. Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy for peptic ulcer disease in Helicobacter
pylori positive patients. Cochrane Database Syst Rev
2006;(2):CD003840. Crossref
29. Moayyedi P, Soo S, Deeks J, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev 2006;2:CD002096. Crossref
30. Zullo A, Hassan C, Andriani A, et al. Eradication therapy
for Helicobacter pylori in patients with gastric MALT
lymphoma: a pooled data analysis. Am J Gastroenterol
2009;104:1932-8. Crossref
31. Hong Kong Cancer Registry, Hospital Authority, Hong Kong SAR Government. 10 most common cancers in Hong
Kong in 2020. Available from: https://www3.ha.org.hk/cancereg/default.asp. Accessed 19 Jun 2023.
32. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the
Maastricht V/Florence Consensus Report. Gut 2017;66:6-30. Crossref
33. Du Y, Zhu H, Liu J, et al. Consensus on eradication of Helicobacter pylori and prevention and control of gastric cancer in China (2019, Shanghai). J Gastroenterol Hepatol
2020;35:624-9. Crossref
34. Holster IL, Valkhoff VE, Kuipers EJ, Tjwa ET. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology
2013;145:105-12.e15. Crossref
35. Lanas Á, Carrera-Lasfuentes P, Arguedas Y, et al. Risk of upper and lower gastrointestinal bleeding in patients taking nonsteroidal anti-inflammatory drugs, antiplatelet
agents, or anticoagulants. Clin Gastroenterol Hepatol
2015;13:906-12.e2. Crossref
36. Chan FK, Ching JY, Suen BY, Tse YK, Wu JC, Sung JJ. Effects of Helicobacter pylori infection on long-term risk of peptic ulcer bleeding in low-dose aspirin users. Gastroenterology
2013;144:528-35. Crossref
37. Ng JC, Yeomans ND. Helicobacter pylori infection and the risk of upper gastrointestinal bleeding in low dose aspirin users: systematic review and meta-analysis. Med J Aust
2018;209:306-11. Crossref
38. Venerito M, Schneider C, Costanzo R, Breja R, Röhl FW, Malfertheiner P. Contribution of Helicobacter pylori
infection to the risk of peptic ulcer bleeding in patients
on nonsteroidal anti-inflammatory drugs, antiplatelet
agents, anticoagulants, corticosteroids and selective
serotonin reuptake inhibitors. Aliment Pharmacol Ther
2018;47:1464-71.Crossref
39. Sostres C, Carrera-Lasfuentes P, Benito R, et al. Peptic ulcer bleeding risk. The role of Helicobacter pylori infection in NSAID/low-dose aspirin users. Am J Gastroenterol
2015;110:684-9. Crossref
40. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: treatment of Helicobacter pylori
infection. Am J Gastroenterol 2017;112:212-39. Crossref
41. Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64:1353-67. Crossref
42. Queiroz DM, Harris PR, Sanderson IR, et al. Iron status and Helicobacter pylori infection in symptomatic
children: an international multi-centered study. PLoS One
2013;8:e68833. Crossref
43. Yuan W, Li Y, Yang K, et al. Iron deficiency anemia in Helicobacter pylori infection: meta-analysis of randomize Crossref
44. Qu XH, Huang XL, Xiong P, et al. Does Helicobacter pylori infection play a role in iron deficiency anemia? A meta-analysis. World J Gastroenterol 2010;16:886-96. Crossref
45. Xia W, Zhang X, Wang J, Sun C, Wu L. Survey of anaemia and Helicobacter pylori infection in adolescent girls in Suihua, China and enhancement of iron intervention effects by H pylori eradication. Br J Nutr 2012;108:357-62. Crossref
46. Hudak L, Jaraisy A, Haj S, Muhsen K. An updated systematic review and meta-analysis on the association
between Helicobacter pylori infection and iron deficiency
anemia. Helicobacter 2017;22:e12330. Crossref
47. Stasi R, Sarpatwari A, Segal JB, et al. Effects of eradication
of Helicobacter pylori infection in patients with immune
thrombocytopenic purpura: a systematic review. Blood
2009;113:1231-40. Crossref
48. Arnold DM, Bernotas A, Nazi I, et al. Platelet count response to H pylori treatment in patients with immune thrombocytopenic purpura with and without H pylori
infection: a systematic review. Haematologica 2009;94:850-6. Crossref
49. Ikuse T, Toda M, Kashiwagi K, et al. Efficacy of Helicobacter pylori eradication therapy on platelet recovery in pediatric simmune thrombocytopenic purpura—case series and a
systematic review. Microorganisms 2020;8:1457. Crossref
50. Jones NL, Koletzko S, Goodman K, et al. Joint ESPGHAN/NASPGHAN Guidelines for the Management of
Helicobacter pylori in Children and Adolescents (Update
2016). J Pediatr Gastroenterol Nutr 2017;64:991-1003. Crossref
51. Kato S, Shimizu T, Toyoda S, et al. The updated JSPGHAN guidelines for the management of Helicobacter pylori infection in childhood. Pediatr Int 2020;62:1315-31. Crossref
52. Calvet X, Sánchez-Delgado J, Montserrat A, et al. Accuracy of diagnostic tests for Helicobacter pylori: a reappraisal. Clin Infect Dis 2009;48:1385-91. Crossref
53. Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection—a critical review. Aliment Pharmacol Ther 2004;20:1001-17. Crossref
54. Makristathis A, Barousch W, Pasching E, et al. Two enzyme
immunoassays and PCR for detection of Helicobacter pylori
in stool specimens from pediatric patients before and after
eradication therapy. J Clin Microbiol 2000;38:3710-4. Crossref
55. Feldman RA, Deeks JJ, Evans SJ. Multi-laboratory comparison of eight commercially available Helicobacter pylori serology kits. Helicobacter pylori Serology Study
Group. Eur J Clin Microbiol Infect Dis 1995;14:428-33. Crossref
56. Dechant FX, Dechant R, Kandulski A, et al. Accuracy of different rapid urease tests in comparison with histopathology in patients with endoscopic signs of gastritis. Digestion 2020;101:184-90. Crossref
57. Fallone CA, Chiba N, van Zanten SV, et al. The Toronto Consensus for the Treatment of Helicobacter pylori
infection in adults. Gastroenterology 2016;151:51-69.e14. Crossref
58. Graham DY, Moss SF. Antimicrobial susceptibility testing for Helicobacter pylori is now widely available: when, how, why. Am J Gastroenterol 2022;117:524-8. Crossref
59. Shah SC, Iyer PG, Moss SF. AGA Clinical Practice Update on the management of refractory Helicobacter pylori infection: expert review. Gastroenterology 2021;160:1831-41. Crossref
60. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol
Hepatol 2018;16:800-8.e7. Crossref
61. Tang HL, Li Y, Hu YF, Xie HG, Zhai SD. Effects of CYP2C19 loss-of-function variants on the eradication of
H pylori infection in patients treated with proton pump
inhibitor–based triple therapy regimens: a meta-analysis
of randomized clinical trials. PLoS One 2013;8:e62162. Crossref
62. Kuo YT, Liou JM, El-Omar EM, et al. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis. Lancet Gastroenterol
Hepatol 2017;2:707-15. Crossref
63. Guo CG, Jiang F, Cheung KS, Li B, Ooi PH, Leung WK. Timing of prior exposure to antibiotics and failure of Helicobacter pylori eradication: a population-based study. J Antimicrob Chemother 2022;77:517-23. Crossref
64. Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev 2013;(12):CD008337. Crossref
65. Liu KS, Hung IF, Seto WK, et al. Ten day sequential versus 10 day modified bismuth quadruple therapy as empirical
firstline and secondline treatment for Helicobacter pylori
in Chinese patients: an open label, randomised, crossover
trial. Gut 2014;63:1410-5. Crossref
66. Hung IF, Chan P, Leung S, et al. Clarithromycin-amoxycillin-containing triple therapy: a valid empirical
first-line treatment for Helicobacter pylori eradication in
Hong Kong? Helicobacter 2009;14:505-11. Crossref
67. Salazar CO, Cardenas VM, Reddy RK, Dominguez DC, Snyder LK, Graham DY. Greater than 95% success with
14-day bismuth quadruple anti–Helicobacter pylori
therapy: a pilot study in US Hispanics. Helicobacter
2012;17:382-90. Crossref
68. Yang JC, Lin CJ, Wang HL, et al. High-dose dual therapy is superior to standard first-line or rescue therapy for
Helicobacter pylori infection. Clin Gastroenterol Hepatol
2015;13:895-905.e5. Crossref
69. Gao CP, Zhang D, Zhang T, et al. PPI-amoxicillin dual therapy for Helicobacter pylori infection: an update based on a systematic review and meta-analysis. Helicobacter
2020;25:e12692. Crossref
70. Xin Y, Manson J, Govan L, et al. Pharmacological regimens for eradication of Helicobacter pylori: an overview of systematic reviews and network meta-analysis. BMC
Gastroenterol 2016;16:80. Crossref
71. Marin AC, McNicholl AG, Gisbert JP. A review of rescue regimens after clarithromycin-containing triple therapy
failure (for Helicobacter pylori eradication). Expert Opin
Pharmacother 2013;14:843-61. Crossref
72. Gisbert JP, Morena F. Systematic review and meta-analysis: levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment Pharmacol Ther
2006;23:35-44. Crossref
73. Saad RJ, Schoenfeld P, Kim HM, Chey WD. Levofloxacin-based triple therapy versus bismuth-based quadruple therapy for persistent Helicobacter pylori infection: a metaanalysis.
Am J Gastroenterol 2006;101:488-96. Crossref
74. Gisbert JP, Calvet X. Review article: rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther 2012;35:209-21. Crossref
75. Gisbert JP, Castro-Fernandez M, Perez-Aisa A, et al. Fourth-line rescue therapy with rifabutin in patients with
three Helicobacter pylori eradication failures. Aliment
Pharmacol Ther 2012;35:941-7. Crossref
76. Van der Poorten D, Katelaris PH. The effectiveness of rifabutin triple therapy for patients with difficult-to-eradicate Helicobacter pylori in clinical practice. Aliment
Pharmacol Ther 2007;26:1537-42. Crossref
77. Shi X, Zhang J, Mo L, Shi J, Qin M, Huang X. Efficacy and safety of probiotics in eradicating Helicobacter
pylori: a network meta-analysis. Medicine (Baltimore)
2019;98:e15180. Crossref
78. Gingold-Belfer R, Niv Y, Schmilovitz-Weiss H, Levi Z, Boltin D. Susceptibility-guided versus empirical treatment
for Helicobacter pylori infection: a systematic review and
meta-analysis. J Gastroenterol Hepatol 2021;36:2649-58. Crossref
79. Yaghoobi M, Farrokhyar F, Yuan Y, Hunt RH. Is there an increased risk of GERD after Helicobacter pylori
eradication? A meta-analysis. Am J Gastroenterol
2010;105:1006-14. Crossref
80. Saad AM, Choudhary A, Bechtold ML. Effect of Helicobacter pylori treatment on gastroesophageal reflux
disease (GERD): meta-analysis of randomized controlled
trials. Scand J Gastroenterol 2012;47:129-35. Crossref
81. Tan J, Wang Y, Sun X, Cui W, Ge J, Lin L. The effect of Helicobacter pylori eradication therapy on the
development of gastroesophageal reflux disease. Am J Med
Sci 2015;349:364-71. Crossref
82. Upala S, Sanguankeo A, Saleem SA, Jaruvongvanich V. Effects of Helicobacter pylori eradication on insulin resistance and metabolic parameters: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2017;29:153-9. Crossref
83. Nwokolo CU, Freshwater DA, O’Hare P, Randeva HS. Plasma ghrelin following cure of Helicobacter pylori. Gut 2003;52:637-40. Crossref
84. Furuta T, Shirai N, Xiao F, Takashima M, Hanai H. Effect of s infection and its eradication on nutrition. Aliment Pharmacol Ther 2002;16:799-806. Crossref
85. Liou JM, Chen CC, Chang CM, et al. Long-term changes of gut microbiota, antibiotic resistance, and metabolic parameters after Helicobacter pylori eradication: a multicentre, open-label, randomised trial. Lancet Infect Dis 2019;19:1109-20. Crossref
86. Lane JA, Murray LJ, Harvey IM, Donovan JL, Nair P, Harvey RF. Randomised clinical trial: Helicobacter pylori eradication is associated with a significantly increased
body mass index in a placebo-controlled study. Aliment
Pharmacol Ther 2011;33:922-9. Crossref