Hong Kong Med J 2023 Dec;29(6):506–13 | Epub 4 Dec 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Uveal and conjunctival melanomas: disease course and outcomes in Chinese patients
Julia YY Chan, FCOphthHK, FHKAM (Ophthalmology)1,2; Stacey Carolyn Lam, FCOphthHK, FHKAM (Ophthalmology)1,2; Hunter KL Yuen, FRCOphth, FRCSEd1,2
1 Hong Kong Eye Hospital, Hong Kong SAR, China
2 Department of Ophthalmology and Visual Sciences, The Chinese University of Hong Kong, Hong Kong SAR, China
Corresponding author: Dr Julia YY Chan (yy.chan@link.cuhk.edu.hk)
Abstract
Introduction: Epidemiological studies of ocular
melanomas have largely focused on Caucasian
populations. This study reviewed the course and
outcomes of uveal melanoma (UM) and conjunctival
melanoma (CM) in Chinese patients.
Methods: This retrospective study included patients
with UM and CM who received treatment in a
tertiary eye centre in Hong Kong from January 1994
to December 2019. Data were recorded concerning
patient demographics, tumour laterality, tumour
characteristics, investigations performed, treatment
regimen, and final outcomes.
Results: During the 25-year study period, there
were 13 patients with UM and 11 patients with CM
who did not display nodal or systemic involvement
at diagnosis. The mean ± standard deviation ages
at diagnosis of UM and CM were 59 ± 15.8 and
57 ± 13.9 years, respectively. There were more men
among patients with UM than among those with
CM (P=0.042). Most patients with UM underwent
primary enucleation (n=12; 92.3%), whereas most
patients with CM underwent orbital exenteration (n=9; 81.8%). The prognosis was significantly worse
for CM than for UM. The median disease-free
survival were 5.2 years (range, 0.7-20.5) and 2.1
years (range, 0.1-24.9) for UM and CM, respectively.
Melanoma-related mortality was significantly higher
among patients with CM than among those with UM
(P=0.006).
Conclusion: Compared with UM, CM has higher
rates of systemic metastasis and tumour-related
mortality in Hong Kong Chinese patients, regardless
of prior definitive treatment.
New knowledge added by this study
- Among Chinese patients, conjunctival melanoma constituted a larger proportion of ocular melanoma cases than previously identified in Caucasian populations, similar to the findings in a Korean study.
- Compared with uveal melanoma, conjunctival melanoma has a worse prognosis with a higher rate of metastasis, shorter disease-free survival, and shorter overall survival.
- Primary enucleation is an effective treatment for patients with uveal melanoma, but patients with conjunctival melanoma may experience systemic metastasis despite radical treatment with wide excisional biopsy or primary orbital exenteration.
- Ophthalmologists and oncologists should offer long-term follow-up with regular systemic surveillance to patients with conjunctival melanoma who have received definitive treatment.
Introduction
Primary ocular melanoma from melanocytes within
the eye constitutes uveal melanoma (UM), whereas
ocular melanoma from melanocytes on the globe
surface is considered conjunctival melanoma (CM).
Although both UM and CM develop from similar
neural crest lineages and are classified as ocular
melanomas, they have distinct clinical behaviours,
management, prognosis, cancer staging features,
and molecular characteristics. Overall, 85% of
melanomas in ocular regions arise from the uvea
(iris, choroidal, and ciliary body), 5% arise from the
conjunctiva, and 10% occur in other sites.1 The most common site of UM is the choroid, which is involved in 90% of cases.2
For most cases of UM, the primary treatment
is enucleation. Alternative options include plaque
brachytherapy and proton beam radiation; these
options are currently unavailable in Hong Kong and
similar Asian countries (eg, Singapore). Systemic
metastases of UM most commonly affect the liver,
followed by lung and bone. Conjunctival melanoma
is usually managed by complete excisional biopsy
with wide surgical margins, using a ‘no-touch’
technique. Adjuvant therapies such as cryotherapy
or topical mitomycin C are offered, followed by reconstruction with an amniotic membrane graft.
Orbital exenteration may be necessary for advanced
tumours where local resection is not feasible.
Metastatic disease, often to regional lymph nodes
and the brain, occurs in 20% to 30% of patients with
CM.3
Melanomas are generally rare tumours and
their incidences are particularly low in Asian
populations. The annual incidence of UM in
Caucasian populations is 5.1 cases per million,2
whereas the respective incidences in Japanese4
and Korean5 populations are 0.2 and 0.6 cases per
million. The incidences of CM are similar: 0.2 to
0.5 cases per million in Caucasian populations3
and 0.15 cases per million in Asian populations.6
Because of this rarity among Asian populations,
very few studies have focused on Chinese patients.
Epidemiological studies have largely involved
Caucasian populations. Clinical characteristics
may differ in Asian populations; for example, Asian
patients are initially diagnosed with UM5 and CM6 at
younger ages.
Known risk factors for UM include fair
skin, light-coloured eyes, congenital ocular
melanocytosis, ocular melanocytoma, and BAP1
tumour predisposition syndrome.7 In contrast,
risk factors for CM include increased conjunctival
pigmentation and a history of primary acquired
melanosis. Predictors of recurrence or new tumour
formation after CM treatment are older age, a history of prior conjunctival surgery, and advanced
T subclassification in the American Joint Committee
on Cancer (AJCC) staging system.8
This study explored the disease course and
outcomes of UM and CM in Chinese patients
without nodal metastasis on presentation.
Methods
This retrospective study included patients who
received treatment for UM or CM in a single
tertiary ophthalmic centre in Hong Kong between
January 1994 and December 2019. Inclusion criteria
included Chinese ethnicity and imaging-confirmed
lack of tumour dissemination at diagnosis. Exclusion
criteria were <6 months of follow-up, insufficient
available information, loss to follow-up, or presence
of any precursor lesions (eg, atypical primary
acquired melanosis). The following data were
recorded: patient demographics, tumour laterality,
tumour characteristics (eg, presentation and staging
according to the AJCC Cancer Staging Manual [8th
Edition]9 10), investigations performed, treatment
regimen, and final outcomes. The study adhered to
the principles outlined in the Declaration of Helsinki.
Provisional clinical diagnoses of UM and
CM were made based on each patient’s medical
history, primary acquired melanosis status, and
clinical presentation. Final diagnoses of UM and
CM were confirmed by excisional biopsy or analysis
of a specimen collected during definitive surgical
treatment. Unless refused by the patient, positron
emission tomography–computed tomography
(PET-CT) scans of UM and CM were performed
after 2001 (when such scans became commercially
available). Magnetic resonance imaging scans of
the brain and orbit, as well as fundus fluorescein
angiography and indocyanine green angiography,
were conducted to investigate suspected UM. All
patients with pathologically confirmed UM or
CM underwent definitive surgical treatment. The
specific surgical treatment was selected according
to melanoma location and size, depth of invasion,
systemic metastasis status, and the patient’s physical
condition.
SPSS software (Windows version 20; IBM
Corp, Armonk [NY], US) was utilised for statistical
analysis. Differences between patient groups were
calculated using the Chi squared and Mann-Whitney
U tests. P values <0.05 were considered statistically
significant. Kaplan-Meier survival analysis was
performed to estimate disease-free survival and
overall survival in patients who had received
definitive treatment. Continuous data were reported
as mean ± standard deviation.
Results
In this 25-year retrospective study, there were 13 and 11 patients with pathologically confirmed UM and
CM, respectively. Two other patients were excluded:
both displayed ocular symptoms (upper eyelid
mass and bloody discharge) but were subsequently
diagnosed with ocular metastases of primary nasal
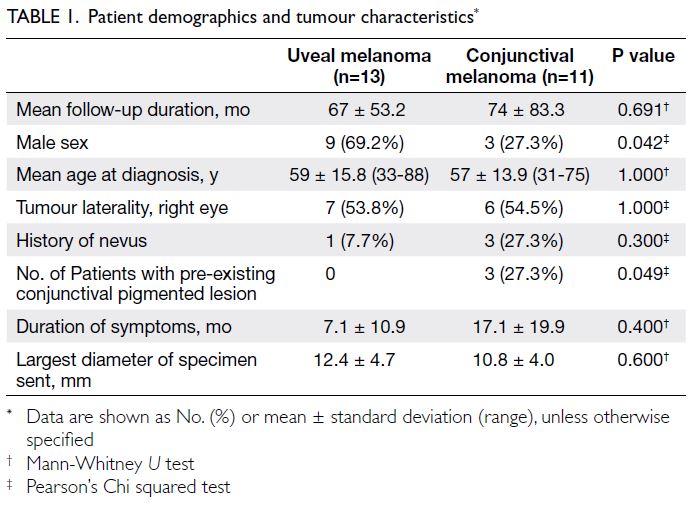
melanoma. The mean follow-up durations for UM
and CM were 67 ± 53.2 and 74 ± 83.3 months,
respectively. There were more men among patients
with UM than among those with CM (P=0.042). The
incidence of pre-existing conjunctival nevus was
higher among patients with CM than among those
with UM (P=0.049). There were no other significant
differences in terms of age at diagnosis, tumour
laterality, duration of symptoms, history of ocular
nevi, or pathologically determined lesion diameter
(Table 1).
Twenty-three of the 24 included patients had
visual symptoms on presentation. Among patients
with UM, 84.6% (n=11) reported blurring of vision
and 7.7% (n=1) presented with photopsia. In one male
patient (7.7%), the tumour was discovered during
a routine ophthalmological examination. Patients
with CM had more diverse visual symptoms: 63.6%
(n=7) exhibited conjunctival pigmentation, 18.2%
(n=2) had either an upper or lower eyelid mass, 9.1%
(n=1) displayed bloody ocular discharge, and 9.1%
(n=1) had experienced bleeding from the mass.
Tumour staging
Retrospective melanoma staging of UM9 and CM10
was conducted in accordance with the AJCC Cancer
Staging Manual (8th Edition). Uveal melanoma
stages varied from T1a to T4a and from stage I to
IIIa. Most UM cases were clinical stage II: 38.5%
(n=5), 38.5% (n=5), 7.7% (n=1), and 15.3% (n=2) of
patients with UM had clinical stage I, IIA, IIB, and
IIIA tumours, respectively. Pathological staging of
the tumours revealed that 46.2% (n=6) were spindle
cell type, 38.5% (n=5) were epithelioid cell type, and
the remaining 15.3% (n=2) were mixed cell type
(ie, >10% epithelioid cells and <90% spindle cells).
Pathological staging tended to be equivalent to or
higher than the initial clinical staging: two patients
with pathological stage IIB disease were initially
diagnosed with clinical stage I disease, and three
patients with pathological stage IIB disease were
initially diagnosed with clinical stage IIA disease.
For the remaining eight patients, the clinical and
pathological stages were identical. Overall, 23.1%
(n=3), 15.4% (n=2), 46.2% (n=6), and 15.3% (n=2) of
patients with UM had pathological stage I, IIA, IIB
and III tumours, respectively.
At diagnosis of CM, the clinical T (cT) stage
was cT2 in 72.7% (n=8) of patients and cT3 in 27.3%
(n=3) of patients. There were no cT1 or cT4 tumours
in our cohort. The pathological T (pT) stage was pT2
in 54.5% (n=6) of patients and pT3 in 45.5% (n=5)
of patients. No patients were diagnosed with pT1 or pT4 disease. Unlike the approach or UM, the AJCC
staging system for CM does not include guidance
regarding overall stage; there is only clinical and
pathological staging for the T (tumour) component.
One patient had a lower clinical stage (T2b) than
pathological stage (T3b); all other patients had
identical clinical and pathological stages.
Disease management
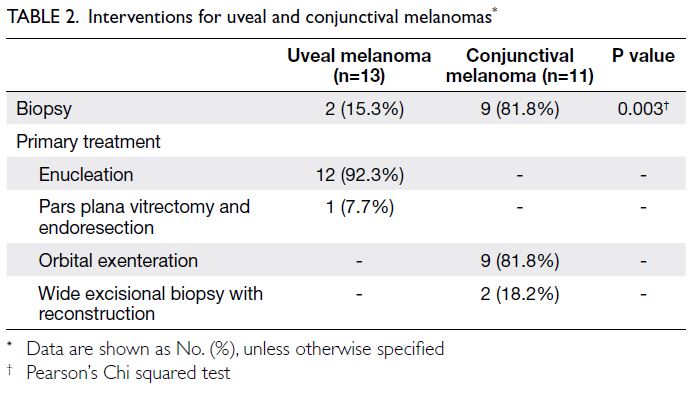
In terms of disease management, biopsies were more
frequently performed before definitive treatment in
patients with CM than in those with UM (P=0.003).
Enucleation was performed in 92.3% (n=12) of
patients with UM, whereas orbital exenteration was
performed in 81.8% (n=9) of patients with CM. The
interventions and treatments performed are shown
in Table 2.
After definitive treatment, all patients initially
attended weekly follow-up visits; they gradually
transitioned to follow-up at 6-month intervals. Clinical examinations were performed for any
surgical complications or disease recurrence. In
cases of suspected disease recurrence or metastasis,
contrast CT scans of the brain and orbit were
performed. No patients with UM or CM had short-term
or long-term wound complications. There were
no instances of local recurrence during follow-up.
One patient with UM (7.7%) had metastasis
to bone, lung, and breast tissue, as determined by
high-resolution CT at 105 months of follow-up.
The patient died 117 months after initial diagnosis.
Two patients (15.3%) died of causes unrelated to
melanoma, such as hepatocellular carcinoma or
squamous cell carcinoma of the lung.
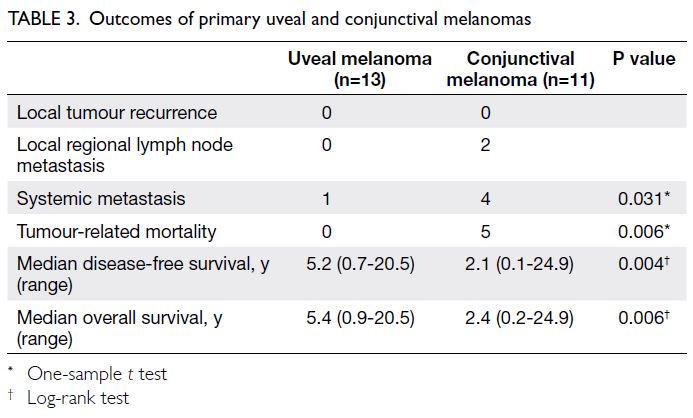
Patients with CM had worse outcomes than
those with had UM, in terms of systemic metastasis
(P=0.031) and tumour-related mortality rates
(P=0.006). Overall, 45.5% (n=4) of patients with
CM developed systemic metastasis during follow-up;
the mean time from definitive treatment to
systemic metastasis was 55.8 ± 66 months (range,
14-168). Cases were detected when patients were
symptomatic and admitted to an acute care hospital
for whole-body PET-CT or brain magnetic resonance
imaging. Lymph node metastasis in two patients
and liver metastasis in one patient were confirmed
by fine needle aspiration cytology. Brain metastasis
was identified by brain CT in two patients, one of
whom had simultaneous bone and lung metastases
confirmed by PET-CT. Overall, 18.2% (n=2) of
patients died of causes unrelated to melanoma. The
mean time from definitive treatment to death was
39 ± 26 months (range, 1-64). Patients with CM
had shorter median disease-free survival (P=0.004)
and overall survival (P=0.006). Detailed results are
presented in Table 3.
At 2 years after definitive treatment,
the probabilities of disease-free survival were
approximately 0.55 for CM and 1.0 for UM. At
10 years, these probabilities decreased to 0.4 for CM and 0.7 for UM. Overall survival was 100%
among patients with UM at 10 years after definitive
treatment. In contrast, patients with CM displayed
a progressive decrease in overall survival, reaching
35% at 5 years after definitive treatment. Survival
curves are depicted in Figures 1 and 2.
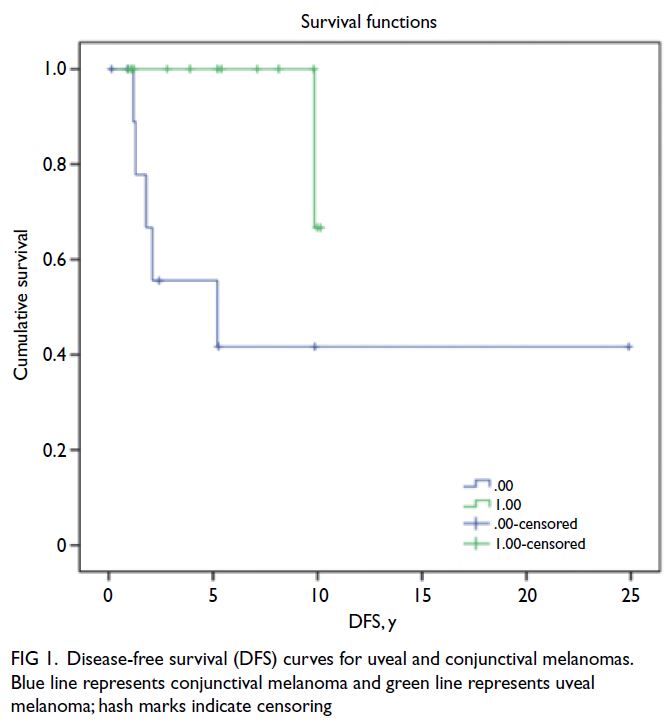
Figure 1. Disease-free survival (DFS) curves for uveal and conjunctival melanomas. Blue line represents conjunctival melanoma and green line represents uveal melanoma; hash marks indicate censoring
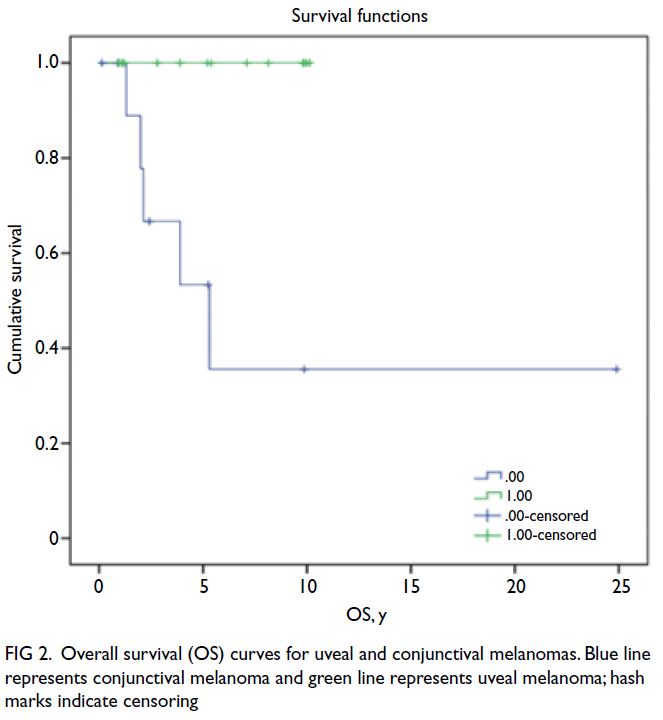
Figure 2. Overall survival (OS) curves for uveal and conjunctival melanomas. Blue line represents conjunctival melanoma and green line represents uveal melanoma; hash marks indicate censoring
Discussion
To our knowledge, this is the first retrospective study
of disease course and outcomes among patients with
UM and CM in southern China.
Demographics
In the present study, the mean ages at diagnosis of
UM and CM were 59 and 57 years, respectively.
These findings are similar to the ages at diagnosis of
UM in Taiwan (55 years)11 and the US (58 years),12
but slightly older than the ages in Singapore (52
years)13 and South Korea (53 years).14 The findings
are also similar to the ages at diagnosis of CM in the
US and mainland China (61 years15 and 54 years,16 respectively). It is clear that UM and CM both affect
patients in their late 50s to early 60s, regardless of
ethnicity.
Uveal melanoma primarily affected men in the
present study, consistent with findings in Australian17
and European18 Caucasian populations. No previous
studies have identified oestrogen receptors in
normal uveal tissue or UM tumours,19 and there is no
evidence that oral contraceptives or postmenopausal
oestrogens participate in UM aetiology.20 Because
female hormones do not have protective or
exacerbating roles in UM, there is speculation that
testosterone receptors are present on UM tumours,
leading to a higher incidence in men.21 The present
study revealed that CM primarily affected women,
but previous studies have not found a relationship
between sex and CM incidence.22 23 In contrast to our
results, a study in mainland China showed that CM
primarily affected men.15 Further studies are needed
regarding the relationship between sex and CM.
Studies in Caucasian populations have
demonstrated that the incidence of UM is much
higher than that of CM, with a ratio of approximately
3 to 1.21 24 In the present study, CM constituted a
larger proportion of ocular melanoma cases than
previously identified in Caucasian populations, with
results similar to epidemiological findings from
Korea.5 Asian populations may have a lower risk of
UM, but further research is warranted to confirm
this speculation.
Clinical presentation
Zloto et al21 described an intriguing phenomenon
whereby men were less likely than women to report
symptoms of UM. Among our patients with UM,
nearly all reported symptoms; one male patient did not report symptoms and had a tumour identified
during a routine examination. Although this finding
was not statistically significant, it is consistent with
the previous results in a Caucasian population.2
In the present study, more patients with CM
had pre-existing conjunctival pigmented lesions,
compared with those who had UM. Somatic
mutations in the BRAF gene frequently occur in
human melanomas, including CM; such mutations
are strongly associated with ultraviolent light
exposure.25 In subtropical regions such as Hong
Kong where there is abundant sunlight, analyses of
underlying BRAF gene mutations could be insightful.
Tumour staging
Among 10 ophthalmology centres on four continents
(North and South America, Europe, and Asia),
the proportions of UM stages I, IIA, IIB, and IIIA
were 32%, 34%, 22.1%, and 8.8%, respectively10; the
present study demonstrated a comparatively greater
proportion of cases with stage IIB or higher.
Comparative analysis of CM data revealed
similar results: patients in the present study had
higher clinical stages relative to patients in the US,
where three-quarters and more than half of the
patients had clinical and pathological stage I disease.
This discrepancy could be explained by the rare
nature of CM, particularly in Asian populations,
leading to lower disease awareness and delayed
referral to an ophthalmologist.
Treatment
Primary enucleation was an effective treatment
for patients with UM in the present study; only
one patient (7.7%) had systemic metastasis. In
that patient, although no systemic metastasis was
detected on PET-CT at diagnosis, the maximum
standardised uptake value of the UM tumour was
particularly high (9.4); UM tumours in other patients
had maximum standardised uptake values of 0 to
3.9. Considering the exceptionally high metabolic
rate in the UM tumour of the patient with systemic
metastasis, microscopic metastasis may have been
present before enucleation.
Despite radical treatment with wide excisional
biopsy or primary orbital exenteration, systemic
metastasis occurred in nearly half of the patients
with CM. Regular imaging surveillance by PET-CT
may be beneficial for patients with CM after orbital
exenteration.
Prognosis
Among patients with UM, we observed a much
lower rate of systemic metastasis than reported in
Singapore (7.7% vs 45.5%26). This difference could
be attributed to the performance of early radical
treatment (ie, enucleation) before detection of systemic metastasis. Notably, the disease-free
survival rate was better in our cohort of patients
with UM than in another study of Chinese patients
with UM (5- and 10-year disease-free survival
rates of 80% and 70%, respectively)27 and better
than in a study of Singaporean patients with UM
(5-year disease-free survival rate of 56.8%).13 For
comparison, in Caucasian populations, the 5-year
and 10-year disease-free survival rates were 81.6%17
and 50%,28 respectively.
The rates of systemic metastasis and tumour-related
mortality are consistently higher in patients
with CM than in those with UM. In the present
study, tumour-related mortality at 5 years was
similar to the rate in Chinese patients (30.5%)16
but higher than that among patients in the US
(7%).29 In five large studies (n=734 cases overall)
of CM after surgical resection with tumour-free
margins, the 5-year overall survival rates ranged
from 74% to 86%.30 31 32 33 34 A recent Singaporean study
revealed a slightly lower 5-year overall survival rate
(68.8%).13 In the present study, the 5-year overall
survival rate was substantially lower than the rates
in other countries. Shields et al29 reported that a
pathologically confirmed positive tumour margin
and the absence of limbal involvement were risk
factors for CM metastasis. Although all patients with
CM in our cohort had pathologically confirmed clear
margins, most patients with metastasis (n=6) had
palpebral CM (83.3%, n=5) in which the melanoma
did not reach the limbus. Thus, tumour location
and ethnicity may explain the poor overall survival
among patients with CM in the present study.
The higher risk of metastasis and lower rates
of 5- and 10-year overall survival in CM, compared
with UM, could be attributed to multiple factors. We
did not find significant differences between UM and
CM in terms of tumour stage or delays in diagnosis/treatment. We suspect that the differences between
tumours are related to the nature of the disease, the
primary mode of metastasis (UM spreads through
the vasculature, whereas CM spreads through the
lymphatic system), and genetic alterations (UM
is associated with chromosomal abnormalities
and CM is associated with mutations in specific
genes). In terms of monitoring UM recurrence,
the use of single-cell technologies to identify
circulating tumour cells has implications for clinical
stratification, particularly in cases of UM where
specific genetic mutations have been identified.35
Because circulating tumour cell tests have received
US Food and Drug Administration’s approval for
clinical use in the management of various tumours
(eg, metastatic breast and prostate cancers), they
may be utilised in future efforts to detect circulating
UM tumour cells.
In our centre, sentinel lymph node biopsy
(SLNB) is not performed as a component of CM management. Mor et al36 recommend SLNB for
patients with CM because false-negative findings are
rare and 5-year survival can reach 79%. Therefore,
early diagnosis of CM, including SLNB in cases
with poor prognosticating factors (lack of limbal
involvement and positive biopsy margin), and
radical neck dissection as appropriate (with support
from head and neck surgeons) should be considered
before systemic treatment is offered.
Limitations
This retrospective study included a low number
of patients. Additionally, genetic testing was not
performed on tissue samples from patients with
pathologically confirmed tumours. Chromosomal
abnormalities (monosomy 3, gain of chromosome
8q, and monosomy 3 combined with loss of 1p36)
have been associated with decreased survival in
UM,37 whereas mutations in the BRAF, RAS, cKIT,
and NF1 genes have been associated with CM38;
thus, survival could be more closely related to
specific genetic features, and it may be inappropriate
to consider these tumours as single entities.
Conclusion
Because UM and CM are rare conditions, they
represent challenges for primary physicians
(ie, timely referral) and ophthalmologists (ie,
appropriate treatment and adequate long-term
follow-up). Currently, there is limited information
regarding the roles of newer targeted therapies for
UM and CM, compared with the application of such
therapies to cutaneous melanoma. Among patients
with CM, long-term mortality remains high despite
definitive radical treatment. This study explored the
disease course and outcomes in Hong Kong Chinese
patients, then compared the findings with data from
patients in other countries. For patients with UM
and CM, we recommend long-term follow-up with
close monitoring, a detailed medical history, holistic
assessment involving cervical and head lymph node
palpation, and ophthalmological examination.
Collaborations with oncologists to provide regular
systemic evaluation during long-term followup,
with the goal of early detection for distant
metastases, are also important. Chest X-ray, brain
magnetic resonance imaging, and cytology with
SLNB should be performed regularly (annually if
possible) to improve survival, particularly in patients
with CM.
Author contributions
Concept or design: HKL Yuen.
Acquisition of data: JYY Chan.
Analysis or interpretation of data: SC Lam, JYY Chan.
Drafting of the manuscript: JYY Chan.
Critical revision of the manuscript for important intellectual content: SC Lam, HKL Yuen.
Acquisition of data: JYY Chan.
Analysis or interpretation of data: SC Lam, JYY Chan.
Drafting of the manuscript: JYY Chan.
Critical revision of the manuscript for important intellectual content: SC Lam, HKL Yuen.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The study protocol was approved by the Research Ethics
Committee (Kowloon Central/Kowloon East) of Hospital
Authority, Hong Kong (Ref No.: KC/KE-21-0129/ER-1).
Patients were treated in accordance with the tenets of
the Declaration of Helsinki, provided written informed
consent for all treatments and procedures, and consented to
publication of this report.
References
1. Chang AE, Karnell LH, Menck HR. The National Cancer
Data Base report on cutaneous and noncutaneous
melanoma: a summary of 84,836 cases from the past
decade. The American College of Surgeons Commission
on Cancer and the American Cancer Society. Cancer
1998;83:1664-78. Crossref
2. Kaliki S, Shields CL. Uveal melanoma: relatively rare but
deadly cancer. Eye (Lond) 2017;31:241-57. Crossref
3. Vora GK, Demirci H, Marr B, Mruthyunjaya P. Advances
in the management of conjunctival melanoma. Surv
Ophthalmol 2017;62:26-42. Crossref
4. Stang A, Parkin DM, Ferlay J, Jöckel KH. International
uveal melanoma incidence trends in view of a decreasing
proportion of morphological verification. Int J Cancer
2005;114:114-23. Crossref
5. Park SJ, Oh CM, Kim BW, Woo SJ, Cho H, Park KH.
Nationwide incidence of ocular melanoma in South Korea
by using the national cancer registry database (1999-2011).
Invest Ophthalmol Vis Sci 2015;56:4719-24. Crossref
6. Hu DN, Yu G, McCormick SA, Finger PT. Population-based
incidence of conjunctival melanoma in various races
and ethnic groups and comparison with other melanomas.
Am J Ophthalmol 2008;145:418-23. Crossref
7. Jager MJ, Shields CL, Cebulla CM, et al. Uveal melanoma.
Nat Rev Dis Primers 2020;6:24. Crossref
8. Vaidya S, Dalvin LA, Yaghy A, et al. Conjunctival
melanoma: risk factors for recurrent or new tumor in
540 patients at a single ocular oncology center. Eur J
Ophthalmol 2021;31:2675-85. Crossref
9. Baron ED, Di Nicola M, Shields CL. Updated AJCC
classification for posterior uveal melanoma. Available
from: https://retinatoday.com/pdfs/0518RT_Oncology.pdf. Accessed 1 Mar 2021.
10. Jain P, Finger PT, Damato B, et al. Multicenter, international
assessment of the Eighth Edition of the American Joint
Committee on Cancer Cancer Staging Manual for
conjunctival melanoma. JAMA Ophthalmol 2019;137:905-11. Crossref
11. Ma ST, Hsieh YT, Wei YH, Liao SL. A 45-year experience
of uveal melanoma in Taiwan: verification of American
Joint Committee on Cancer staging system and prognostic factors. J Formos Med Assoc 2021;120:1361-8. Crossref
12. Shields CL, Furuta M, Thangappan A, et al. Metastasis
of uveal melanoma millimeter-by-millimeter in 8033
consecutive eyes. Arch Ophthalmol 2009;127:989-98. Crossref
13. Tan LL, Hong J, Goh WL, et al. Clinical features and
survival outcomes of ocular melanoma in a multi-ethnic
Asian cohort. Sci Rep 2020;10:16367. Crossref
14. Lee CS, Lee J, Choi JJ, et al. Cytogenetics and prognosis
for uveal melanoma in Korean patients. Acta Ophthalmol
2011;89:e310-4. Crossref
15. Yousef YA, Finger PT. Predictive value of the seventh
edition American Joint Committee on Cancer staging
system for conjunctival melanoma. Arch Ophthalmol
2012;130:599-606. Crossref
16. Zhou C, Wang Y, Jia R, Fan X. Conjunctival melanoma in
Chinese patients: local recurrence, metastasis, mortality,
and comparisons with Caucasian patients. Invest
Ophthalmol Vis Sci 2017;58:5452-9. Crossref
17. Vajdic CM, Kricker A, Giblin M, et al. Incidence of ocular
melanoma in Australia from 1990 to 1998. Int J Cancer
2003;105:117-22. Crossref
18. Singh AD, Turell ME, Topham AK. Uveal melanoma: trends
in incidence, treatment, and survival. Ophthalmology
2011;118:1881-5. Crossref
19. Mäkitie T, Tarkkanen A, Kivelä T. Comparative
immunohistochemical oestrogen receptor analysis in
primary and metastatic uveal melanoma. Graefes Arch
Clin Exp Ophthalmol 1998;236:415-9. Crossref
20. Holly EA, Aston DA, Ahn DK, Kristiansen JJ, Char DH.
Uveal melanoma, hormonal and reproductive factors in
women. Cancer Res 1991;51:1370-2.
21. Zloto O, Pe’er J, Frenkel S. Gender differences in clinical
presentation and prognosis of uveal melanoma. Invest
Ophthalmol Vis Sci 2013;54:652-6. Crossref
22. McLaughlin CC, Wu XC, Jemal A, Martin HJ, Roche LM,
Chen VW. Incidence of noncutaneous melanomas in the
U.S. Cancer 2005;103:1000-7. Crossref
23. Wong JR, Nanji AA, Galor A, Karp CL. Management of
conjunctival malignant melanoma: a review and update.
Expert Rev Ophthalmol 2014;9:185-204. Crossref
24. Hu DN, Yu GP, McCormick SA, Schneider S, Finger PT.
Population-based incidence of uveal melanoma in various
races and ethnic groups. Am J Ophthalmol 2005;140:612-7. Crossref
25. Besaratinia A, Pfeifer GP. Sunlight ultraviolet irradiation
and BRAF V600 mutagenesis in human melanoma. Hum
Mutat 2008;29:983-91. Crossref
26. Wong W, Sundar G, Chee C, Zhao PS, Rajagopalan R,
Gopal L. Clinical spectrum, treatment and outcomes
of uveal melanoma in a tertiary centre. Singapore Med J
2019;60:474-8. Crossref
27. Yue H, Qian J, Yuan Y, et al. Clinicopathological
characteristics and prognosis for survival after enucleation
of uveal melanoma in Chinese patients: long-term follow-up.
Curr Eye Res 2017;42:759-65. Crossref
28. Virgili G, Gatta G, Ciccolallo L, et al. Survival in patients
with uveal melanoma in Europe. Arch Ophthalmol
2008;126:1413-8. Crossref
29. Shields CL, Shields JA, Gündüz K, et al. Conjunctival
melanoma: risk factors for recurrence, exenteration,
metastasis, and death in 150 consecutive patients. Arch
Ophthalmol 2000;118:1497-507. Crossref
30. Tuomaala S, Eskelin S, Tarkkanen A, Kivelä T. Population-based assessment of clinical characteristics predicting
outcome of conjunctival melanoma in whites. Invest
Ophthalmol Vis Sci 2002;43:3399-408.
31. Missotten GS, Keijser S, De Keizer RJ, De Wolff-Rouendaal
D. Conjunctival melanoma in the Netherlands: a
nationwide study. Invest Ophthalmol Vis Sci 2005;46:75-82. Crossref
32. Shields CL, Kaliki S, Al-Dahmash SA, Lally SE, Shields JA.
American Joint Committee on Cancer (AJCC) clinical
classification predicts conjunctival melanoma outcomes.
Ophthal Plast Reconstr Surg 2012;28:313-23. Crossref
33. Esmaeli B, Wang X, Youssef A, Gershenwald JE. Patterns
of regional and distant metastasis in patients with
conjunctival melanoma: experience at a cancer center over
four decades. Ophthalmology 2001;108:2101-5. Crossref
34. Werschnik C, Lommatzsch PK. Long-term follow-up of patients with conjunctival melanoma. Am J Clin Oncol
2002;25:248-55. Crossref
35. Wang MM, Chen C, Lynn MN, et al. Applying single-cell
technology in uveal melanomas: current trends and
perspectives for improving uveal melanoma metastasis
surveillance and tumor profiling. Front Mol Biosci
2021;7:611584. Crossref
36. Mor JM, Rokohl AC, Koch KR, Heindl LM. Sentinel lymph
node biopsy in the management of conjunctival melanoma:
current insights. Clin Ophthalmol 2019;13:1297-302. Crossref
37. van den Bosch T, Kilic E, Paridaens D, de Klein A. Genetics
of uveal melanoma and cutaneous melanoma: two of a
kind? Dermatol Res Pract 2010;2010:360136. Crossref
38. Gkiala A, Palioura S. Conjunctival melanoma: update on
genetics, epigenetics and targeted molecular and immune-based
therapies. Clin Ophthalmol 2020;14:3137-52. Crossref