Hong Kong Med J 2023 Aug;29(4):359.e1-3 | Epub 14 Jun 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PICTORIAL MEDICINE
A curious case of early-onset dementia
Whitney CT Ip, MRCP (UK)1; YF Shea, FHKAM (Medicine)1; TK Ling, MHKCPath2; CY Law, FHKAM (Pathology)2; CW Lam, FHKAM (Pathology)2,3; Patrick KC Chiu, FHKAM (Medicine)1
1 Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Hong Kong SAR, China
2 Division of Chemical Pathology, Department of Pathology, Queen Mary Hospital, Hong Kong SAR, China
3 Department of Pathology, The University of Hong Kong, Hong Kong SAR, China
Corresponding author: Dr YF Shea (syf321@ha.org.hk)
A 63-year-old woman was referred to the memory
clinic of Queen Mary Hospital, Hong Kong in
September 2021 for early-onset dementia (defined
as onset before age of 65 years). Three months
previously, she had developed stepwise deterioration
in cognitive function and self-care ability following
a right occipital lobe infarction. After a course of
rehabilitation, her Montreal Cognitive Assessment–Hong Kong version and the Barthel Index scores were 5/30 and 9/20, respectively. The Montreal
Cognitive Assessment–Hong Kong version score
would have remained below the 2nd percentile
even if the visual components, affected due to
her potential visual deficit from stroke, had been
excluded from the total score. A detailed review
of her medical history revealed that she had had
progressive anterograde amnesia since the age of
56 years. She worked previously as a professional accountant but had retired since the onset of
cognitive impairment. Within 1 year, she developed
executive dysfunction and personality change with
aggressiveness. She reportedly had disorientation,
prosopagnosia, and apraxia prior to her stroke in
2021. 18F-fluorodeoxyglucose positron emission
tomography–computed tomography and Pittsburgh
Compound B positron emission tomography at
the age of 61 years, before the episode of stroke,
showed bilateral temporoparietal hypometabolism
(Fig 1) and diffuse amyloid load, especially over
bilateral frontal lobes, parietal lobes, and posterior
cingulate gyrus (Fig 2). The imaging findings were
compatible with Alzheimer’s disease (AD). Further
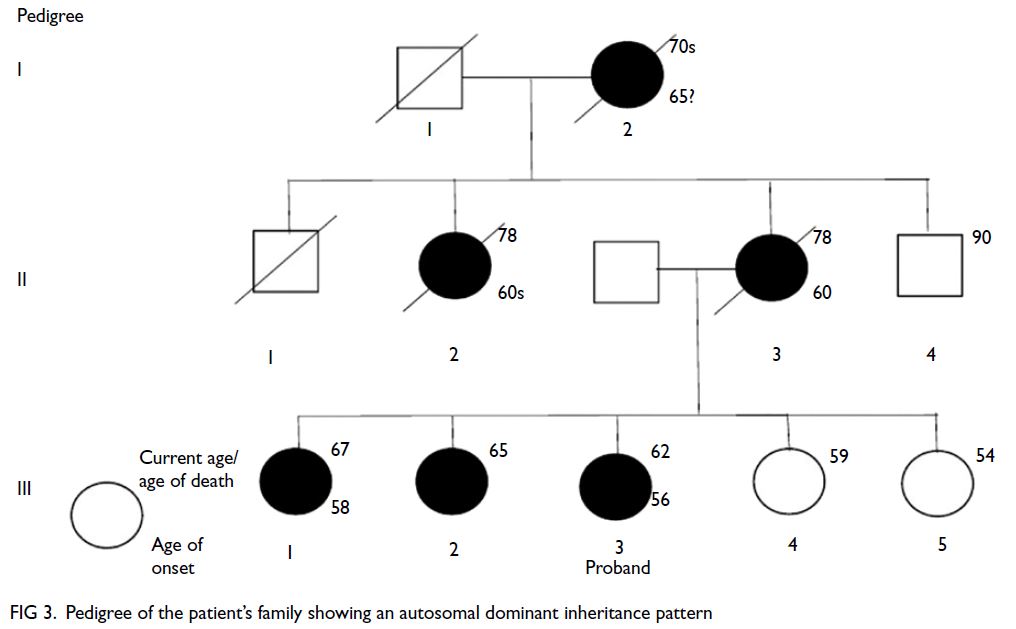
examination of her family history revealed multiple
first-degree relatives with early-onset dementia with
an autosomal dominant inheritance pattern (Fig 3).
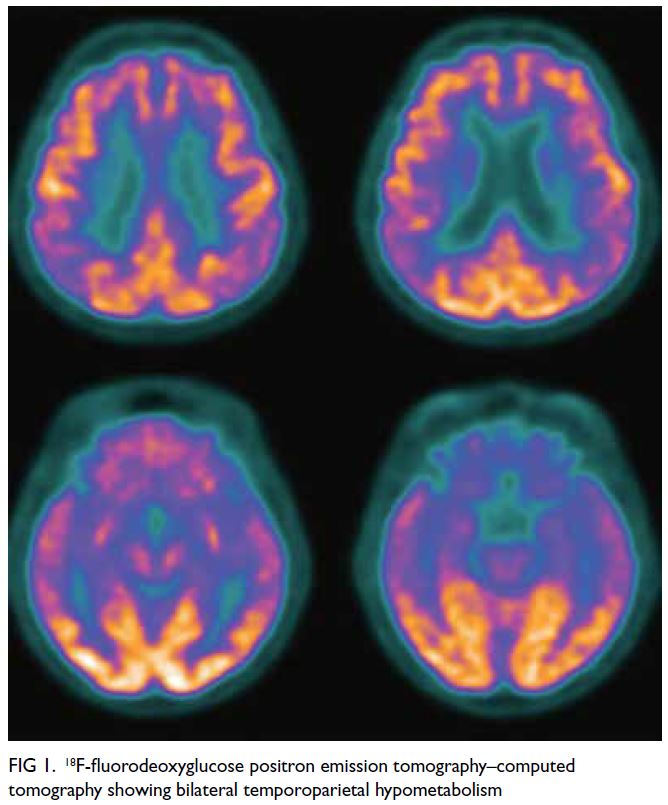
Figure 1. 18F-fluorodeoxyglucose positron emission tomography–computed tomography showing bilateral temporoparietal hypometabolism
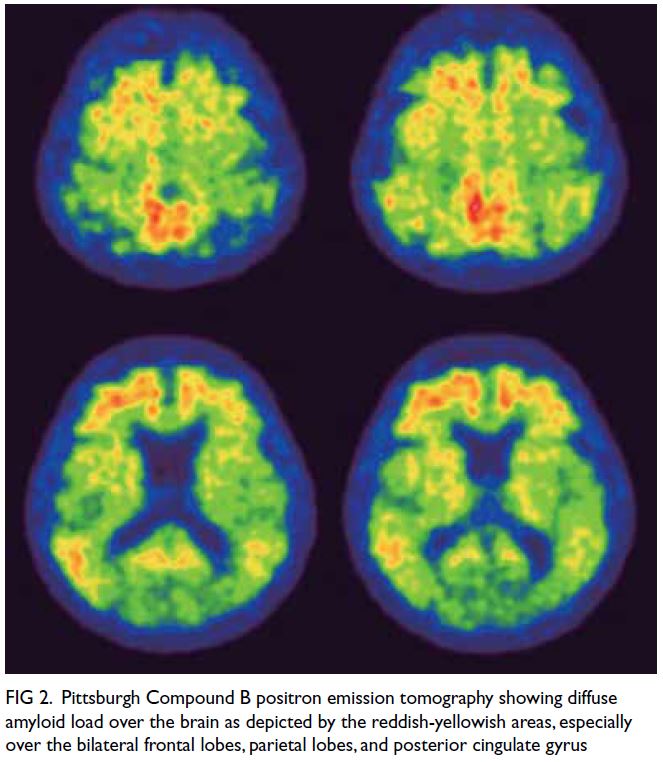
Figure 2. Pittsburgh Compound B positron emission tomography showing diffuse amyloid load over the brain as depicted by the reddish-yellowish areas, especially over the bilateral frontal lobes, parietal lobes, and posterior cingulate gyrus
Given the strong family history of early-onset
dementia, familial AD gene panel, which included
amyloid protein precursor (APP), presenilin-1
(PSEN1), and presenilin-2 (PSEN2), by next-generation
sequencing was performed. Presenilin-1
was positive for a heterozygous mutation with
a missense variant (c.786G>C, p.Leu262Phe),
confirming the diagnosis of familial AD. No known
pathogenic variants were detected in APP or
PSEN2 genes. The family was referred for genetic
counselling.
Familial AD accounts for less than 0.5% of
early-onset AD cases.1 It is caused by mutations in the PSEN1, PSEN2 or APP gene, resulting in early deposition of amyloid plaques due to
overproduction and deposition of Aβ42 leading to
early neurodegeneration (the amyloid hypothesis).1
Nonetheless a newer presenilin hypothesis suggests
alternative mechanisms, eg, loss-of-function
of PSEN1 with suppressed γ-secretase activity
and increased Aβ42/Aβ40 ratios, resulting in
neurodegeneration.2 Presenilin-1 mutations account
for up to 71.5% of Asian cases of familial AD.1 These patients may have an atypical presentation such as
parkinsonism or spastic paraparesis.1 With a few
exceptions, familial AD mutations are considered
fully penetrant with the development of dementia
at a predictable age. Families should be referred
for genetic counselling since carriers may have half
the chance of transmitting the mutation to a child.
Carriers may be referred to a tertiary centre for
potential pre-implantation genetic testing. There
have been three reported families in Hong Kong
with familial AD and different mutations.3 4 Patients with PSEN1 p.Leu262Phe tend to have a decreased
word-finding ability.5
In summary, familial AD should be considered
when a patient presents with early-onset cognitive
impairment and a strong family history of early-onset
dementia. Referral to chemical pathologists
for genetic testing is important for family planning
and advance care planning.
Author contributions
All authors contributed to the concept or design of the study, acquisition of the data, analysis or interpretation of the
data, drafting of the manuscript, and critical revision of the
manuscript for important intellectual content. All authors
had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki. Patient consent was obtained for all investigations including treatment, procedures, and publication.
References
1. Shea YF, Chu LW, Chan AO, Ha J, Li Y, Song YQ. A systematic review of familial Alzheimer’s disease: differences
in presentation of clinical features among three mutated
genes and potential ethnic differences. J Formos Med Assoc
2016;115:67-75. Crossref
2. Kelleher RJ 3rd, Shen J. Presenilin-1 mutations and Alzheimer’s disease. Proc Natl Acad Sci U S A 2017;114:629-31. Crossref
3. Shea YF, Chan AO, Chu LW, et al. Novel presenilin 1 mutation (p.F386I) in a Chinese family with early-onset Alzheimer’s disease. Neurobiol Aging 2017;50:168.e9-11. Crossref
4. Shea YF, Chu LW, Chan AO, Kwan JS. Delayed diagnosis of an old Chinese woman with familial Alzheimer’s disease. J Formos Med Assoc 2015;114:1020-1. Crossref
5. Forsell C, Froelich S, Axelman K, et al. A novel pathogenic mutation (Leu262Phe) found in the presenilin 1 gene in early-onset Alzheimer’s disease. Neurosci Lett 1997;234:3-6. Crossref