Hong Kong Med J 2023 Aug;29(4):330–6 | Epub 21 Jul 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Comparison of United Kingdom and United States screening criteria for detecting retinopathy of prematurity in Hong Kong
Lawrence PL Iu, FHKAM (Ophthalmology), MPH (HK)1; Wilson WK Yip, FHKAM (Ophthalmology)1; Julie YC Lok, FHKAM (Ophthalmology)1; Mary Ho, FHKAM (Ophthalmology)1; Leanne TY Cheung, MB, BS2; Tania HM Wu, MB, BS3; Alvin L Young, FHKAM (Ophthalmology), FRCOphth1
1 Department of Ophthalmology and Visual Sciences, Prince of Wales Hospital, Hong Kong SAR, China
2 Department of Ophthalmology, Tung Wah Eastern Hospital, Hong Kong SAR, China
3 Department of Paediatrics and Adolescent Medicine, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
Corresponding author: Dr Lawrence PL Iu (dr.lawrenceiu@gmail.com)
Abstract
Introduction: We examined whether the United
Kingdom (UK) or the United States (US) screening
criteria are more appropriate for retinopathy of
prematurity (ROP) screening in Hong Kong, in
terms of sensitivity for detecting type 1 ROP and the
number of infants requiring screening.
Methods: In this retrospective cohort study, we
reviewed the medical records of all infants who
underwent ROP screening from 2009 to 2018 at a
tertiary hospital in Hong Kong. During this period,
all infants born at gestational age (GA) ≤31 weeks
and 6 days or birth weight (BW) <1501 g (ie, the
UK screening criteria) underwent ROP screening.
We determined the number of infants requiring
screening and the number of type 1 ROP cases that
would have been missed if the US screening criteria
(GA ≤30 weeks & 0 days or BW ≤1500 g) had been
used.
Results: Overall, 796 infants were screened using the
UK screening criteria. If the US screening criteria
had been used, the number of infants requiring
screening would have decreased by 21.1%; all type
1 ROP cases would have been detected (38/38,
100% sensitivity). Of the 168 infants who would not
have been screened using the US screening criteria, only four of them (2.4%) had developed ROP (all maximum stage 1 only).
Conclusion: In our population, the use of the US screening criteria could reduce the number of infants
screened without compromising sensitivity for the
detection of type 1 ROP requiring treatment. We
suggest narrowing the GA criterion for consistency
with the US screening criteria during ROP screening
in Hong Kong.
New knowledge added by this study
- In our population, the use of the United States (US) screening criteria, instead of the United Kingdom (UK) criteria, could reduce the number of infants requiring retinopathy of prematurity (ROP) screening by 21.1%.
- The use of the US screening criteria would have detected 100% of type 1 ROP cases over a 10-year period, compared with the UK screening criteria, indicating that the US screening criteria would not compromise sensitivity for the detection of type 1 ROP requiring treatment in Hong Kong.
- There is a need to consider narrowing the gestational age criterion for consistency with the US screening criteria during ROP screening in Hong Kong.
- A review of published literature indicates that our screening outcomes considerably differ from findings in other Asian countries, suggesting that our results are not generalisable to regions outside of Hong Kong.
Introduction
Retinopathy of prematurity (ROP) is a proliferative
retinal vascular disease that affects premature
infants.1 Infants born at low gestational age (GA)
and/or low birth weight (BW) have a risk of ROP.2
Without timely intervention, severe ROP can
progress to retinal detachment and blindness.
Currently, ROP is one of the leading preventable causes of childhood blindness worldwide.3
Successful management of ROP relies on
appropriate screening for early detection of high-risk
disease, along with prompt treatment to prevent
disease progression and visual loss. The United
Kingdom (UK) Guidelines (published in 2008 by the
Royal College of Paediatrics and Child Health, the
Royal College of Ophthalmologists, and the British Association of Perinatal Medicine) recommend
that all infants born at GA ≤31 weeks and 6 days or
BW <1501 g undergo ROP screening.4 On the other
hand, the United States (US) Guidelines (published
in 2013 and 2018 by the American Academy of
Pediatrics, American Academy of Ophthalmology,
and American Association for Pediatric
Ophthalmology and Strabismus) use narrower
criteria; they recommend that all infants born at GA
≤30 weeks and 0 days or BW ≤1500 g undergo ROP
screening.5 6
In Hong Kong, many hospitals use the UK
screening criteria to guide ROP detection.7 8 9
Although the UK screening criteria are appropriate
for ROP detection in many countries,10 11 12 they are
not universally appropriate.13 14 15 16 17 18 In India14 15 19 and
China,17 18 some infants with GA and BW above
the UK screening thresholds also developed severe
ROP requiring treatment. Thus, there is a need to
understand the epidemiology of ROP in Hong Kong
and evaluate the utility of current international
guidelines for ROP detection in Hong Kong infants.
In the Early Treatment for Retinopathy of
Prematurity study,20 type 1 ROP was defined as: (1)
zone I, any stage of ROP, with plus disease; (2) zone
I, stage 3 ROP, without plus disease; or (3) zone II,
stage 2 or 3 ROP, with plus disease. Type 1 ROP
requires treatment.4 6 Although it is important not to miss any infants who develop type 1 ROP requiring
treatment, it is also important to avoid unnecessarily
screening a large number of infants because the
ROP screening procedure is painful and distressful
for premature infants; it can lead to oxygen
desaturation, tachycardia, and apnea.2 21 22 There
is also a need to limit the systemic absorption of
dilating eye drops that may cause adverse events.23 24
An effective strategy would reduce the number of
infants unnecessarily screened without missing any
cases of severe ROP requiring treatment. This study
was conducted to determine whether the UK or the
US screening criteria are more appropriate for Hong
Kong, in terms of sensitivity for detecting type 1
ROP and the number of infants requiring screening.
Methods
Patients
In this retrospective cohort study, we reviewed
the medical records of all premature infants who
underwent ROP screening between 1 January 2009
and 31 December 2018 in Prince of Wales Hospital,
Hong Kong. During the study period, all infants born
at GA ≤31 weeks and 6 days or BW <1501 g (ie, UK
screening criteria) underwent ROP screening. Infants
with GA and BW above the UK screening threshold
who had a high risk of ROP because of an unstable
clinical course also underwent ROP screening at
the request of the attending neonatologist. Analyses
were performed to determine the numbers of ROP
and type 1 ROP cases that would have been detected
and missed if the US screening criteria (GA ≤30
weeks & 0 days or BW ≤1500 g) had been used.
All infants who underwent ROP screening in
Prince of Wales Hospital were included. Infants were
excluded if they died or were transferred to other
institutions before completion of ROP screening
without a known ROP outcome. Data were recorded
concerning GA, BW, most severe ROP stage, any
treatment, and treatment outcome. ROP findings
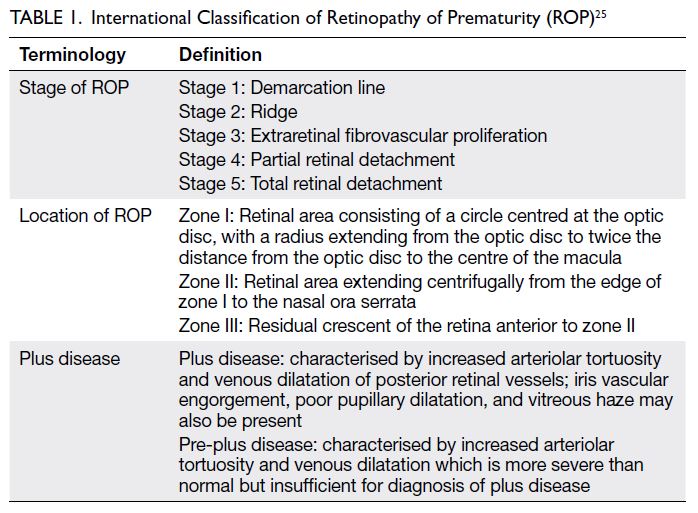
were classified in accordance with the International
Classification of ROP25 (Table 1). Treatment was
indicated for infants with type 1 ROP. If the ROP
stage differed between eyes in an individual infant,
the more severe ROP stage was used for analysis.
Outcome measures and statistical analysis
The primary outcome measure was the sensitivity
of the US screening criteria, compared with the UK
screening criteria, for detection of type 1 ROP. The
secondary outcome measure was the number of
infants requiring screening.
R software (R version 3.6.1) was used for
statistical analysis. All demographic data were
expressed as medians and interquartile ranges
(IQRs).
Results
Demographic data
Of the 857 infants who underwent ROP screening
in the study period, 61 were excluded because they
died or were transferred to other hospitals before the
completion of ROP screening. Thus, the remaining
796 infants (404 boys [50.8%] and 392 girls [49.2%])
were included in the study. The median GA was 30
weeks and 2 days (IQR=7 weeks & 3 days; range,
23 weeks & 4 days to 37 weeks & 4 days), and the
median BW was 1320 g (IQR=471; range, 470-2550).
Incidences of retinopathy of prematurity and
type 1 retinopathy of prematurity
In total, 238 infants (29.9%) developed ROP,
including 38 infants (4.8%) who developed type 1
ROP requiring treatment. The median GA and BW
of infants who developed ROP were 27 weeks and
4 days (IQR=3 weeks & 0 days; range, 23 weeks &
4 days to 35 weeks & 5 days) and 943 g (IQR=366;
range, 470-2550), respectively. The median GA and
BW of infants who developed type 1 ROP were 26
weeks and 0.5 days (IQR=2 weeks & 2.5 days; range,
23 weeks & 4 days to 32 weeks & 0 days) and 781 g
(IQR=315; range, 510-1240), respectively. Among
the infants who developed type 1 ROP requiring
treatment, 81.6% were extremely preterm (GA <28
weeks) infants and 100% were extremely low BW
(<1000 g) infants. Of the treated infants, 13 had stage
2 ROP and 25 had stage 3 ROP. No infants had stage
4 or 5 ROP.
Retinopathy of prematurity cases detected
using the United Kingdom screening criteria
In total, 795 infants underwent ROP screening in
accordance with the UK screening criteria. One
infant had a GA above the UK screening threshold;
however, the infant continued to undergo screening
because he was only 1 day older than the screening
threshold, and the attending neonatologist concluded
that he had a risk of ROP. The UK screening criteria
detected all cases of ROP (n=238) and type 1 ROP
requiring treatment (n=38) [Table 2].
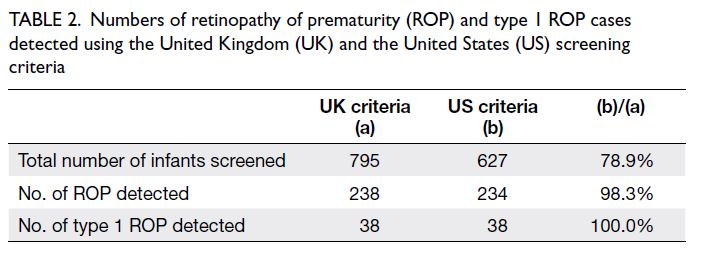
Table 2. Numbers of retinopathy of prematurity (ROP) and type 1 ROP cases detected using the United Kingdom (UK) and the United States (US) screening criteria
Retinopathy of prematurity cases detected
using the United States screening criteria
If the US screening criteria had been used, the
number of infants receiving ROP screening would
have decreased to 627 (21.1% reduction compared
with the UK screening criteria) [Table 2]. The use
of the US screening criteria would have detected
234 cases of ROP (98.3% of cases detected using the
UK criteria, 234/238) and 38 cases of type 1 ROP
(100% of cases detected using the UK criteria, 38/38)
[Table 2]. Of the 168 infants who would not have
been screened using the US screening criteria, only 4 of them (2.4%) had developed ROP (Table 3) and all
cases were mild (maximum stage 1 only); all affected
infants displayed spontaneous resolution of ROP
without the need for treatment. No cases of type 1
ROP were missed by the US screening criteria (ie,
100% sensitivity) [Table 4].
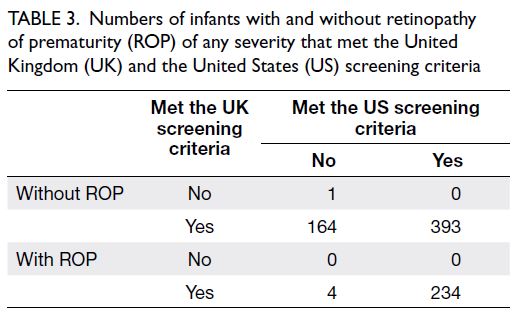
Table 3. Numbers of infants with and without retinopathy of prematurity (ROP) of any severity that met the United Kingdom (UK) and the United States (US) screening criteria
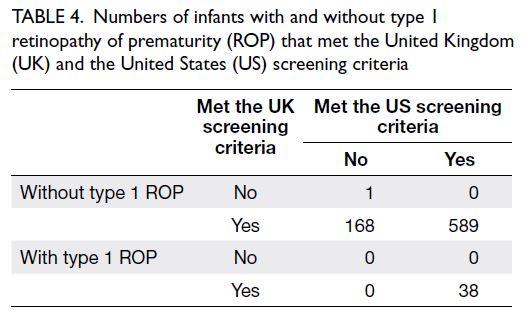
Table 4. Numbers of infants with and without type 1 retinopathy of prematurity (ROP) that met the United Kingdom (UK) and the United States (US) screening criteria
Discussion
This study showed that if the US screening criteria
had been used, instead of the UK screening criteria,
the number of infants screened in our population
would have decreased by 21.1% without missing any
case of type 1 ROP requiring treatment. The number
of ROP cases that would have been missed was very
small (n=4), and all cases were mild (maximum stage
1).
Previous studies showed that many hospitals
in Hong Kong follow the UK screening criteria for
ROP screening7 8 9,26,27; consistent with our findings,
the reported incidences of ROP and type 1 ROP in
Hong Kong were 16% to 28%7 8 9 and 3.4% to 3.8%,7 8 9
respectively. In the present study, type 1 ROP mainly developed in extremely preterm infants with a
median GA of 26 weeks and 0.5 days (IQR=2 weeks
& 2.5 days), suggesting that low GA was an important
predictor of type 1 ROP in our population. Because
the GA criterion is lower in the US screening criteria
(≤30 weeks & 0 days) than in the UK screening
criteria (≤31 weeks & 6 days), the US screening
criteria may be more appropriate for Hong Kong.
Our findings were also consistent with the
results of a study conducted in another hospital in
Hong Kong7; in that study, 12.4% of infants would
not have required ROP screening if the US screening
criteria had been used, rather than the UK criteria,
none of those infants would have developed ROP.
Our results suggest similar outcomes in different
hospitals across Hong Kong.
In a study conducted in Shanghai in mainland
China, the screening thresholds were GA of 34
weeks and BW of 2000 g. The mean GA and BW
of infants requiring ROP treatment were 29.3
weeks (range, 24-35) and 1331 g (range, 750-2550),
respectively17; these infants were more mature and
heavier than the infants in our study. The Shanghai
study showed that 9% of severe ROP cases requiring
treatment would have been missed if the UK
screening criteria were used; 26% would have been
missed if the US screening criteria were used.17
Another study conducted in Beijing in mainland
China showed that 17% of treatment-requiring ROP cases would have been missed if the UK screening
criteria were used; 21% would have been missed if
the US screening criteria were used.18 Therefore,
despite sharing the same Chinese ethnicity, infants
with severe ROP differed in maturity between Hong
Kong and mainland China. This discrepancy could
be the result of variations in comorbidities, perinatal
risk factors, standard of neonatal healthcare, and
level of supplemental oxygen therapy used. Long
oxygen duration, mechanical ventilation, and high
level of supplemental oxygen are known risk factors
for ROP.2 Therefore, the results of our study are not
generalisable to regions outside of Hong Kong.
There is evidence that the UK and the US
screening criteria are not appropriate for many low- and
middle-income countries.15 19 28 29 In North India,
17% of severe ROP cases would have been missed if
the US screening criteria were used; 22% would have
been missed if the UK screening criteria were used.15
In South India, 8% of treatment-requiring ROP cases
would have been missed if the US screening criteria
were used; all of these cases were aggressive posterior
ROP.19 In Saudi Arabia, 35% of infants older than the
UK screening threshold developed ROP; one infant
developed severe ROP (stage 3).28 In Turkey, severe
ROP developed in 3.8% of infants born at ≥32 weeks
and 6.5% of infants born at ≥1500 g.29
Although it is important not to miss any
severe ROP cases, it is also preferable to avoid
missing mild ROP cases because the detection of
early ROP (even mild cases) can influence decisions
regarding systemic management (eg, level of
supplemental oxygen), thereby reducing the rate of
ROP progression. In the present study, only four
cases of mild ROP would have been missed by the
US screening criteria; this number was very small,
compared with the 168 infants (21.1%) who could
have been excluded from screening. The number of
screened infants required to detect one additional
case of ROP was 42 (ie, 168/4). Considering that
few mild ROP cases were missed in exchange for
the exclusion of a large number of infants from
screening, we conclude that it is acceptable and
appropriate to use the US screening criteria for ROP
screening in Hong Kong.
Benefits from reduction in number of
retinopathy of prematurity screening
There are several benefits to reducing the number
of infants screened without compromising the
detection of severe ROP. First, this modified
approach minimises unnecessary stress and the
potential for ROP screening-related adverse events
among infants. Previous studies revealed significant
elevation of blood pressure, increase in pulse rate,
and decrease in oxygen saturation, which persisted
after ROP screening.30 A significant increase in the
number of apnoea events was also observed after screening.31 Approximately half of infants develop
bradycardia from the oculocardiac reflex caused by
scleral depression during screening.32 Second, this
modified approach can reduce hospital expenses. The
estimated cost of ROP screening is approximately
US$230 per infant in the US33 and US$198.9 per
infant in India.34 Third, the approach can reduce
the length of hospitalisation related to delays in
the completion of ROP screening.35 Finally, it may
minimise unnecessary parental stress and anxiety.
For example, one study showed that parents of
infants undergoing ROP screening had significantly
higher anxiety and depression scores compared with
the general population.36
In recent decades, several ROP prediction
models have been developed to improve screening
sensitivity and specificity, including WINROP,37,38
ROPScore,39 CHOP ROP,40 41 CO-ROP,42 STEP-ROP,
43 and G-ROP.44 45 However, these prediction
models have many limitations. First, they require the
collection of postnatal data such as postnatal weight
gain and insulin-like growth factor 1 level, which
may not be available to ophthalmologists. Second,
the mechanisms by which these predictive factors
would interact to affect ROP outcome are not fully
understood. Third, these models were all derived
from Western countries and may not be appropriate
for Asian populations.46 Finally, none of these models
have been validated in Hong Kong. Considering our
findings in the present study, we suggest narrowing
the GA screening criterion to ≤30 weeks and 0 days,
consistent with the US screening criteria; this simple
and straightforward approach avoids the need for
calculations required by prediction models.
Limitations
This study had several limitations. First, its
retrospective design hindered the assessment of
other risk factors (eg, supplemental oxygen level
and comorbidities) that may affect ROP outcomes.
Second, because of the retrospective design, we
could not determine whether the use of a narrower
GA screening criterion would reduce the number
of screenings in real-world clinical practice. A
prospective cohort study is needed to confirm our
findings. Third, although the G-ROP screening
criteria are more sensitive and specific than the
current US screening criteria for populations in
the US,44 45 we could not evaluate the suitability of
G-ROP criteria in our population because we lacked
data concerning postnatal weight gain. Finally, data
were missing regarding infants who died or were
transferred to other hospitals without a known ROP
outcome. Despite these limitations, our findings are
robust because the present study revealed consistent
results when the same screening practices were
applied to a large number of infants over a study
period of 10 years.
Conclusion
Compared with the UK screening criteria, the US
screening criteria appeared to be more appropriate
for our population because they could greatly
reduce the number of infants screened without
compromising sensitivity for the detection of type 1
ROP. Thus, we suggest narrowing the GA criterion
for consistency with the US screening criteria during
ROP screening in Hong Kong. A prospective cohort
study is needed to further explore the impact of
changes to the screening criteria.
Author contributions
Concept or design: LPL Iu, WWK Yip.
Acquisition of data: LPL Iu, LTY Cheung, THM Wu.
Analysis or interpretation of data: LPL Iu, WWK Yip, JYC Lok.
Drafting of the manuscript: LPL Iu.
Critical revision of the manuscript for important intellectual content: WWK Yip, JYC Lok, M Ho, AL Young.
Acquisition of data: LPL Iu, LTY Cheung, THM Wu.
Analysis or interpretation of data: LPL Iu, WWK Yip, JYC Lok.
Drafting of the manuscript: LPL Iu.
Critical revision of the manuscript for important intellectual content: WWK Yip, JYC Lok, M Ho, AL Young.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This research was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research
Ethics Committee (Ref No.: 2020.176) and was performed in
accordance with the tenets of the Declaration of Helsinki. A
waiver of obtaining patient consent has been approved by the
Research Ethics Committee for this retrospective study.
References
1. Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med 2012;367:2515-26. Crossref
2. Kim SJ, Port AD, Swan R, Campbell JP, Chan RV, Chiang MF. Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv Ophthalmol 2018;63:618-37. Crossref
3. Solebo AL, Teoh L, Rahi J. Epidemiology of blindness in children. Arch Dis Child 2017;102:853-7. Crossref
4. Wilkinson AR, Haines L, Head K, Fielder AR. UK retinopathy of prematurity guideline. Early Hum Dev 2008;84:71-4. Crossref
5. Fierson WM; American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2013;131:189-95. Crossref
6. Fierson WM; American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and
Strabismus; American Association of Certified Orthoptists.
Screening examination of premature infants for retinopathy
of prematurity. Pediatrics 2018;142:e20183061. Crossref
7. Iu LP, Lai CH, Fan MC, Wong IY, Lai JS. Screening for
retinopathy of prematurity and treatment outcome in
a tertiary hospital in Hong Kong. Hong Kong Med J
2017;23:41-7. Crossref
8. Yau GS, Lee JW, Tam VT, et al. Incidence and risk factors
of retinopathy of prematurity from 2 neonatal intensive
care units in a Hong Kong Chinese population. Asia Pac J
Ophthalmol (Phila) 2016;5:185-91. Crossref
9. Luk AS, Yip WW, Lok JY, Lau HH, Young AL. Retinopathy
of prematurity: applicability and compliance of guidelines
in Hong Kong. Br J Ophthalmol 2017;101:453-6. Crossref
10. Amer M, Jafri WH, Nizami AM, Shomrani AI, Al-Dabaan AA, Rashid K. Retinopathy of prematurity: are we
missing any infant with retinopathy of prematurity? Br J
Ophthalmol 2012;96:1052-5. Crossref
11. Chaudhry TA, Hashmi FK, Salat MS, et al. Retinopathy of
prematurity: an evaluation of existing screening criteria in
Pakistan. Br J Ophthalmol 2014;98:298-301. Crossref
12. Ugurbas SC, Gulcan H, Canan H, Ankarali H, Torer B, Akova YA. Comparison of UK and US screening criteria
for detection of retinopathy of prematurity in a developing
nation. J AAPOS 2010;14:506-10. Crossref
13. Akman I, Demirel U, Yenice O, Ilerisoy H, Kazokoğlu H,
Ozek E. Screening criteria for retinopathy of prematurity
in developing countries. Eur J Ophthalmol 2010;20:931-7. Crossref
14. Jalali S, Matalia J, Hussain A, Anand R. Modification of
screening criteria for retinopathy of prematurity in India
and other middle-income countries. Am J Ophthalmol
2006;141:966-8. Crossref
15. Vinekar A, Dogra MR, Sangtam T, Narang A, Gupta A.
Retinopathy of prematurity in Asian Indian babies
weighing greater than 1250 grams at birth: ten year data
from a tertiary care center in a developing country. Indian
J Ophthalmol 2007;55:331-6. Crossref
16. Dogra MR, Katoch D, Dogra M. An update on retinopathy
of prematurity (ROP). Indian J Pediatr 2017;84:930-6. Crossref
17. Xu Y, Zhou X, Zhang Q, et al. Screening for retinopathy of
prematurity in China: a neonatal units-based prospective
study. Invest Ophthalmol Vis Sci 2013;54:8229-36. Crossref
18. Chen Y, Li XX, Yin H, et al. Risk factors for retinopathy of
prematurity in six neonatal intensive care units in Beijing,
China. Br J Ophthalmol 2008;92:326-30. Crossref
19. Hungi B, Vinekar A, Datti N, et al. Retinopathy of prematurity in a rural neonatal intensive care unit in South
India—a prospective study. Indian J Pediatr 2012;79:911-5. Crossref
20. Early Treatment for Retinopathy of Prematurity
Cooperative Group. Revised indications for the treatment
of retinopathy of prematurity: results of the early treatment
for retinopathy of prematurity randomized trial. Arch
Ophthalmol 2003;121:1684-94. Crossref
21. Kandasamy Y, Smith R, Wright IM, Hartley L. Pain relief
for premature infants during ophthalmology assessment. J
AAPOS 2011;15:276-80. Crossref
22. Cohen AM, Cook N, Harris MC, Ying GS, Binenbaum G.
The pain response to mydriatic eyedrops in preterm
infants. J Perinatol 2013;33:462-5. Crossref
23. Mitchell A, Hall RW, Erickson SW, Yates C, Lowery S, Hendrickson H. Systemic absorption of cyclopentolate and adverse events after retinopathy of prematurity exams.
Curr Eye Res 2016;41:1601-7. Crossref
24. Alpay A, Canturk Ugurbas S, Aydemir C. Efficiency and
safety of phenylephrine and tropicamide used in premature
retinopathy: a prospective observational study. BMC
Pediatr 2019;19:415. Crossref
25. International Committee for the Classification of
Retinopathy of Prematurity. The International Classification
of Retinopathy of Prematurity revisited. Arch Ophthalmol
2005;123:991-9. Crossref
26. Chow PP, Yip WW, Ho M, Lok JY, Lau HH, Young AL. Trends in the incidence of retinopathy of prematurity over
a 10-year period. Int Ophthalmol 2019;39:903-9. Crossref
27. Yau GS, Lee JW, Tam VT, Liu CC, Chu BC, Yuen CY.
Incidence and risk factors for retinopathy of prematurity in
extreme low birth weight Chinese infants. Int Ophthalmol
2015;35:365-73. Crossref
28. Binkhathlan AA, Almahmoud LA, Saleh MJ, Srungeri S.
Retinopathy of prematurity in Saudi Arabia: incidence, risk
factors, and the applicability of current screening criteria.
Br J Ophthalmol 2008;92:167-9. Crossref
29. Araz-Ersan B, Kir N, Akarcay K, et al. Epidemiological
analysis of retinopathy of prematurity in a referral centre in
Turkey. Br J Ophthalmol 2013;97:15-7. Crossref
30. Jiang JB, Zhang ZW, Zhang JW, Wang YL, Nie C, Luo XQ. Systemic changes and adverse effects induced by retinopathy of prematurity screening. Int J Ophthalmol 2016;9:1148-55.
31. Mitchell AJ, Green A, Jeffs DA, Roberson PK. Physiologic effects of retinopathy of prematurity screening
examinations. Adv Neonatal Care 2011;11:291-7. Crossref
32. Schumacher AC, Ball ML, Arnold AW, Grendahl RL, Winkle RK, Arnold RW. Oculocardiac reflex during ROP
exams. Clin Ophthalmol 2020;14:4263-9. Crossref
33. Yanovitch TL, Siatkowski RM, McCaffree M, Corff KE. Retinopathy of prematurity in infants with birth
weight>or=1250 grams-incidence, severity, and screening
guideline cost-analysis. J AAPOS 2006;10:128-34. Crossref
34. Kelkar J, Kelkar A, Sharma S, Dewani J. A mobile team for screening of retinopathy of prematurity in India: cost-effectiveness, outcomes, and impact assessment. Taiwan J
Ophthalmol 2017;7:155-9. Crossref
35. Zupancic JA, Ying GS, de Alba Campomanes A, Tomlinson LA, Binenbaum G; G-ROP Study Group.
Evaluation of the economic impact of modified screening
criteria for retinopathy of prematurity from the Postnatal
Growth and ROP (G-ROP) study. J Perinatol 2020;40:1100-8. Crossref
36. Xie W, Liang C, Xiang D, Chen F, Wang J. Resilience,
anxiety and depression, coping style, social support
and their correlation in parents of premature infants
undergoing outpatient fundus examination for retinopathy
of prematurity. Psychol Health Med 2021;26:1091-9. Crossref
37. Löfqvist C, Andersson E, Sigurdsson J, et al. Longitudinal postnatal weight and insulin-like growth factor I
measurements in the prediction of retinopathy of
prematurity. Arch Ophthalmol 2006;124:1711-8. Crossref
38. Löfqvist C, Hansen-Pupp I, Andersson E, et al. Validation
of a new retinopathy of prematurity screening method
monitoring longitudinal postnatal weight and insulinlike
growth factor I. Arch Ophthalmol 2009;127:622-7. Crossref
39. Eckert GU, Fortes Filho JB, Maia M, Procianoy RS. A predictive score for retinopathy of prematurity in very low birth weight preterm infants. Eye (Lond) 2012;26:400-6. Crossref
40. Binenbaum G, Ying GS, Quinn GE, et al. A clinical
prediction model to stratify retinopathy of prematurity risk
using postnatal weight gain. Pediatrics 2011;127:e607-14. Crossref
41. Binenbaum G, Ying GS, Quinn GE, et al. The CHOP
postnatal weight gain, birth weight, and gestational age
retinopathy of prematurity risk model. Arch Ophthalmol
2012;130:1560-5. Crossref
42. Cao JH, Wagner BD, McCourt EA, et al. The Colorado-retinopathy of prematurity model (CO-ROP): postnatal weight gain screening algorithm. J AAPOS 2016;20:19-24. Crossref
43. Ricard CA, Dammann CE, Dammann O. Screening tool for early postnatal prediction of retinopathy of prematurity
in preterm newborns (STEP-ROP). Neonatology 2017;112:130-6. Crossref
44. Binenbaum G, Bell EF, Donohue P, et al. Development of
modified screening criteria for retinopathy of prematurity:
primary results from the postnatal growth and retinopathy
of prematurity study. JAMA Ophthalmol 2018;136:1034-40. Crossref
45. Binenbaum G, Tomlinson LA, de Alba Campomanes AG,
et al. Validation of the postnatal growth and retinopathy
of prematurity screening criteria. JAMA Ophthalmol
2019;138:31-7. Crossref
46. Iu LP, Yip WW, Lok JY, Fan MC, Lai CH, Ho M, Young AL.
Prediction model to predict type 1 retinopathy of
prematurity using gestational age and birth weight (PW-ROP).
Br J Ophthalmol 2023;107:1007-11. Crossref