Hong Kong Med J 2023 Aug;29(4):287–94 | Epub 6 Jul 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Awareness, perceptions, and acceptance of human papillomavirus vaccination among parents in Hong Kong
Eddy WH Lam, MB, BS, MMedSc1,2; Hextan YS Ngan, MD, FRCOG1,3; KY Kun, MB, BS, MRCOG1; Dominic FH Li, MB, BS, MRCOG1; WY Wan, MRCP, FHKCCM1; Paul KS Chan, MD, FRCPath1,4
1 HPV Prevention Alliance, Hong Kong SAR, China
2 Department of Otorhinolaryngology, Head and Neck Surgery, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
3 Department of Obstetrics and Gynaecology, Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
4 Department of Microbiology, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
Corresponding author: Prof Paul KS Chan (paulkschan@cuhk.edu.hk)
Abstract
Introduction: This study investigated the awareness, perceptions, and acceptance of human papillomavirus (HPV) vaccination for children among parents in Hong Kong. It also explored factors associated with, and differences in, vaccine acceptance and hesitancy between parents of girls and boys.
Methods: Parents of boys or girls in Primary 5 to 6 were invited to participate in an online survey through an established health and lifestyle e-platform.
Results: Overall, 851 parents completed the survey:
419 (49.2%) had daughters, 348 (40.9%) had sons,
and 84 (9.9%) had children of both genders. Parents
who enrolled their children into the Childhood
Immunisation Programme were more likely to
accept HPV vaccination (79.7% vs 33.7%, odds ratio
[OR]=7.70; 95% confidence interval [CI]=5.39-11.01;
P<0.001); parents of girls were more likely to accept
than parents of boys (86.0% vs 71.8%, OR=2.40; 95%
CI=1.67-3.46; P<0.001). Among parents of girls
and boys, the main reasons for HPV vaccination
acceptance were prevention of cancers (girls: 68.8%
and boys: 68.7%), prevention of sexually transmitted
diseases (girls: 67.3% and boys: 68.3%), and optimal
timing before initiation of sexual activity (girls:
62.8% and boys: 59.8%). Vaccine hesitancy was mainly associated with concerns about serious side-effects
(girls: 66.7% and boys: 68.0%) and the belief
that their children were too young (girls: 60.0% and
boys: 54.0%).
Conclusion: Parents in Hong Kong are hesitant
about HPV vaccination for their sons. This barrier
could be removed by providing information to
correct vaccine safety misconceptions and offering a
gender-neutral vaccination programme through the
school-based Childhood Immunisation Programme.
New knowledge added by this study
- Awareness and acceptance of human papillomavirus (HPV) vaccination for children is lower among parents of boys than among parents of girls in Hong Kong.
- Parental misconceptions regarding vaccine safety and ideal vaccination age are major barriers to HPV vaccination for children.
- The availability of no-cost gender-neutral HPV vaccination would increase parental acceptance.
- The myth that HPV vaccines are unsafe must be dispelled.
- The myth that children in Primary 5 to 6 are too young to receive the HPV vaccine must be dispelled.
Introduction
Human papillomavirus (HPV) is the most frequently
encountered sexually transmitted infection
worldwide1; most men and women become infected with HPV at some stages in their lives.1 Although 90%
of cervical HPV infections spontaneously resolve
within 2 years,2 3 4 persistent infections with high-risk, oncogenic types of HPV can result in invasive cervical cancer. Furthermore, HPV infection is associated with the development of cancers in other locations such as the anus, vulva, vagina, penis, and
oropharynx.5 6 Human papillomavirus vaccination
is a safe and highly effective method for preventing
cervical cancer and other HPV-related cancers.7 8 9 10
Increases in HPV vaccination uptake are
particularly pertinent in Hong Kong, considering that the age-standardised incidence of cervical
cancer increased by an average of 1.2% annually
between 2010 and 2020 (most recent available data).11
In 2020, the age-standardised incidence of cervical
cancer was 7.6 cases per 100 000 women.11 Although
a cervical screening programme for women aged
25-64 years was initiated in Hong Kong in 2004, this
programme functioned primarily as a prospective
record and recall database for women who presented
for screening, rather than a programme for the
proactive inclusion of eligible women.12 Ten years
later, the 2014/15 health survey by the Department
of Health showed that only 59% of women in Hong
Kong had ever been screened for cervical cancer,
and only 47% had been screened within the previous
3 years.13
In Hong Kong, the use of the HPV vaccine was
approved in 2008. Before its incorporation in the
Hong Kong Childhood Immunisation Programme
(CIP), HPV vaccination rates among female students
remained low: 7% to 9% in school-aged girls14 15 16 and
9.7% in university students.17 However, when the HPV vaccine was offered to girls through a no-cost school-based
programme in a feasibility study, the overall
rates of vaccine uptake were 81.4% (1000/1229) for
the first dose and 80.8% (993/1229) for the second
dose.18 These findings were consistent with a report that cost is a major barrier to vaccination.14
The associations of HPV infection with anal, penile, and oropharyngeal cancers, as well as genital
warts,5 6 9 have prompted 57 countries (including
Germany, the United Kingdom, and Australia) to
introduce gender-neutral vaccination into their
national immunisation schedules to provide greater
and more equitable prevention of HPV-related
diseases in their respective populations, with the
implementation as early as 2013 in Austalia.19 20 21
The HPV vaccine uptake in children and
adolescents hinges on parental acceptance. Thus,
this study investigated the awareness, perceptions,
and acceptance of HPV vaccination for children
among parents in Hong Kong. It also explored
factors associated with, and differences in, vaccine
acceptance and hesitancy between parents of girls
and boys.
Methods
Study design
This cross-sectional study was initiated by the HPV
Prevention Alliance, Hong Kong. Representatives
from the HPV Prevention Alliance developed and
approved a structured online questionnaire, which
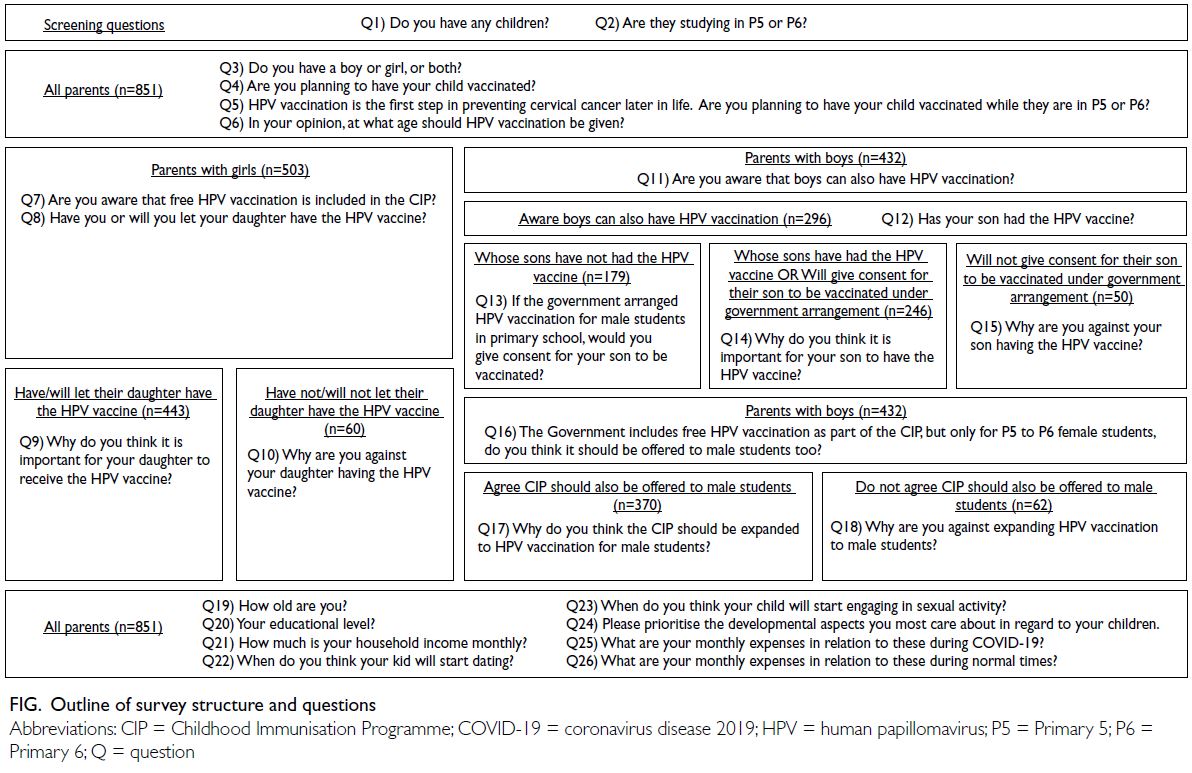
consisted of 26 questions that were designed to
assess parental attitudes towards HPV vaccination
for their children (Fig).
Parents answered general questions about
their children, enrolment in the CIP, and plans to
have their children vaccinated against HPV. At the
time of this survey, only girls were eligible for HPV
vaccination through the no-cost school-based CIP.
Therefore, parents of girls answered questions about
HPV vaccine acceptance/hesitancy and awareness
of HPV inclusion within the CIP. Parents of boys
answered similar questions about HPV vaccine
acceptance/hesitancy, HPV vaccine availability and
appropriateness for boys, and whether inclusion
in the CIP would influence their decision making.
Information was collected regarding parental socio-demographic
and lifestyle characteristics (age,
education level, monthly income, and expenses),
as well as the age at which they expected their
children to begin becoming sexually active. The
survey was developed and conducted in Chinese; it
was translated into English for presentation in this
report.
Data collection
Parents with children in Primary 5 to 6 (aged 10-12
years) were invited to the online questionnaire via
ESDlife, an e-commerce platform that delivers
lifestyle content, products, and services relating
to parenting and health to nearly 1 million people
in Hong Kong. Participants were offered a HK$50
supermarket coupon to encourage completion of the
survey. The survey was conducted between 1 and 7
February 2021.
Statistical analysis
Demographic and lifestyle variables including
age, education level, and monthly income were
expressed as numbers and percentages; these
categorical variables were assessed by the Chi
squared test. Childhood expenses before and during
the coronavirus disease 2019 (COVID-19) pandemic
for education, health, and leisure were regarded as
continuous variables and assessed using t tests. Odds
ratios (ORs) were calculated for various comparison
groups to identify associations with vaccination. P
values <0.05 were considered statistically significant.
All statistical analyses were performed using SPSS
software (Windows version 28; IBM Corp, Armonk
[NY], United States).
Results
In total, 851 parents completed the survey: 419 had
daughters, 348 had sons, and 84 had children of
both genders. There were no missing data because
completion of each question was required before
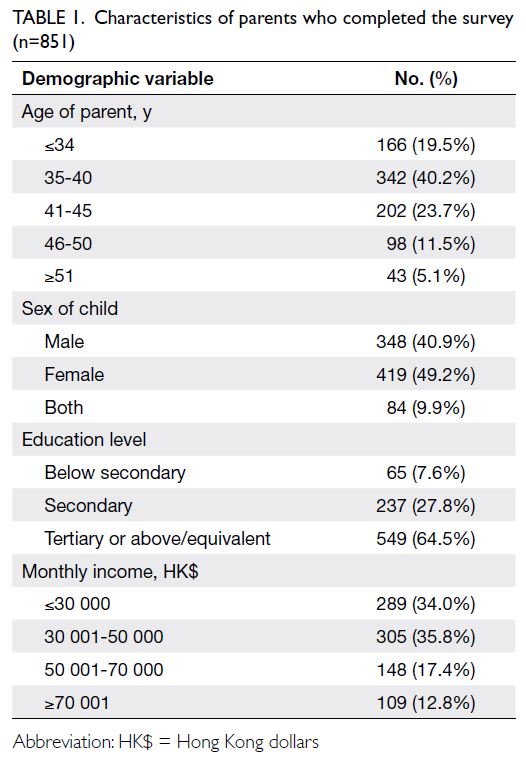
submission of the survey. Most (n=342, 40.2%)
parents were aged between 35 and 45 years, 549
(64.5%) parents had at least tertiary education or
equivalent, and 594 (69.8%) parents had a monthly
income of ≤HK$50 000 (Table 1).
Parents’ characteristics and vaccine acceptance
Parents enrolled in the CIP were more likely to
consent to HPV vaccination, compared with parents
who were not enrolled in the CIP (79.7% vs 33.7%;
OR=7.70, 95% confidence interval [CI]=5.39-11.01,
P<0.001); parents of girls were more likely to consent
than parents of boys (86.0% vs 71.8%; OR=2.40,
95% CI=1.67-3.46, P<0.001). There were no
significant differences between parents who accepted
HPV vaccines and parents who refused HPV
vaccines in terms of age (P=0.522), education level
(P=0.122), or monthly income (P=0.691). Parents
enrolled in the CIP who accepted HPV vaccination
spent significantly less on healthcare, compared
with parents who refused HPV vaccination (mean
[standard deviation, SD] healthcare expenditures:
HK$3246 [6753] vs HK$3853 [7384]; P=0.046).
Overall, 443/503 (88.1%) parents of girls
accepted HPV vaccination; among these 443 parents,
63 (14.2%) had been vaccinated and 380 (85.8%)
were expected to undergo vaccination soon. Parents
who were aware of the government’s no-cost HPV
vaccination programme for Primary 5 to 6 female
students were more likely to have consented (or
planned to consent) to HPV vaccination, compared
with parents who were unaware of the programme
(95.8% vs 79.6%; OR=5.9, 95% CI=2.98-11.61,
P<0.001). Subgroup analysis of parents enrolled
in the CIP (n=420) showed that parents who were
aware of the government’s no-cost HPV vaccination
programme (n=361) were even more likely to accept
HPV vaccination, compared with parents who were
unaware of the programme (n=59) [63.4% vs 10.2%;
OR=15.3, 95% CI=6.41-36.61, P<0.001].
Overall, 296/432 (68.5%) parents of boys were
aware that boys were eligible for HPV vaccination.
Among those 296 parents, only 117 (39.5%) had
consented to HPV vaccination, resulting in an
overall vaccination rate of 27.1% in boys. Among the
179 parents who had not initiated HPV vaccination,
129 (72.1%) stated they would give consent if the
vaccine was provided through a no-cost programme.
Parents who consented to no-cost HPV vaccination
tended to spend less on healthcare both before
and during the COVID-19, compared with parents
who were hesitant to accept HPV vaccination
(mean healthcare expenditures before COVID-19:
HK$3453 [SD=6381] vs HK$6750 [SD=9743],
P<0.001; during COVID-19: HK$2980 [SD=6499]
vs HK$5458 [SD=7969], P=0.009). However, these
subgroups of parents did not significantly differ in
terms of age (P=0.899) or education level (P=0.439).
Drivers and barriers of vaccine acceptance
Most parents indicated that the main reason
for their children to undergo HPV vaccination was prevention of cancers (68.8% and 68.7% for
parents of girls and boys, respectively), followed by
prevention of sexually transmitted diseases (67.3%
and 68.3% for parents of girls and boys, respectively).
They also agreed that the optimal vaccine timing was
before initiation of sexual activity (62.8% and 59.8%
for parents of girls and boys, respectively) [Table 2].
The most common reason for vaccine hesitancy was
the belief that side-effects could occur (66.7% and
68.0% for parents of girls and boys, respectively),
followed by the belief that their children were too
young (60.0% and 54.0% for parents of girls and boys,
respectively), and the belief that the vaccine would
interfere with growth (55.0% and 48.0% for parents
of girls and boys, respectively) [Table 3].
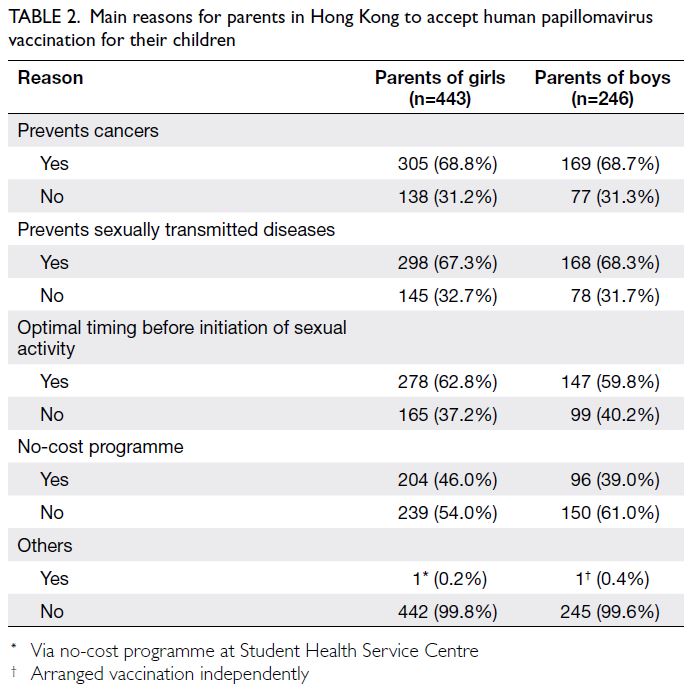
Table 2. Main reasons for parents in Hong Kong to accept human papillomavirus vaccination for their children
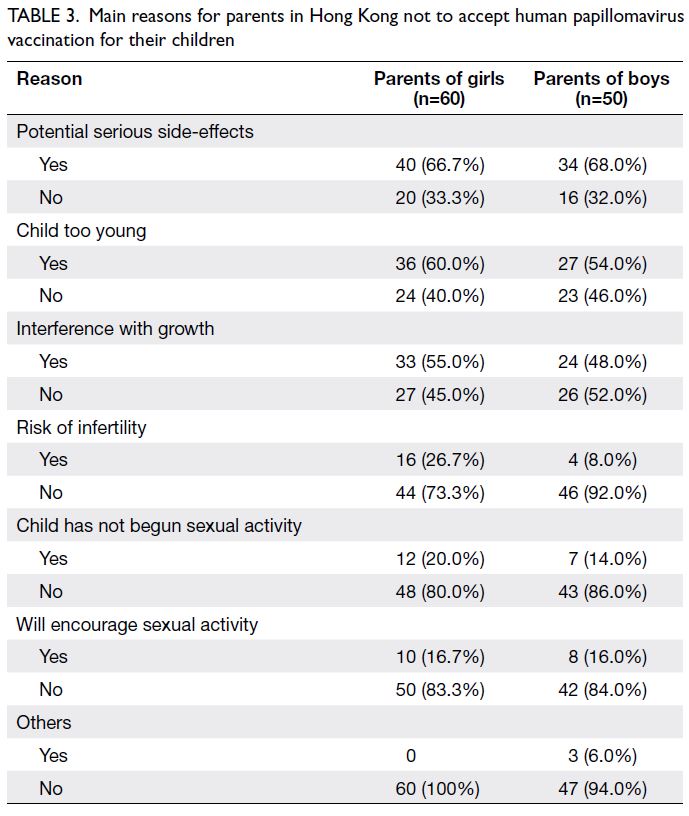
Table 3. Main reasons for parents in Hong Kong not to accept human papillomavirus vaccination for their children
Perceived age of sexual debut
Most parents (40.5%) reported expecting their child
to begin dating at the age of 15 to 17 years, although
36.8% stated that they did not expect their child to
date until at least 18 years of age; 4.9% stated that
their child was dating before 9 years of age. Notably,
1.5% of parents reported their child had begun sexual
activity before the age of 9 years; the corresponding
percentages were 4.3% at the age of 9 to 14 years,
14.4% at the age of 15 to 17 years, and 79.8% at 18
years or older.
Discussion
To our knowledge, this is the first study to explore
awareness, acceptance, and hesitancy in relation
to HPV vaccination for children among parents
of boys and girls in Hong Kong. Despite high
awareness of HPV vaccination for girls, only 12.5%
(63/503) of parents had consented to vaccination
for their daughters at the time of the survey;
another 75.5% (380/503) of parents planned to
consent to vaccination soon. The resulting overall
acceptance rate for parents of girls in Hong Kong
(88.1%) is consistent with a report by Yuen et al,18
which described acceptance rates of 81.4% (for the
first dose) and 80.8% (for the second dose). The
acceptance rates revealed in the present study and
the study by Yuen et al18 are considerably lower than
the overall acceptance rate of 98% for CIP vaccines
among children in Hong Kong.22 There is a need to understand the barriers to HPV vaccination that
affect >10% of parents in Hong Kong.
Barriers to vaccination
The two most common factors associated with
HPV vaccine hesitancy among parents in this study
were ‘potential serious side-effects’ and ‘child too
young’. The notion of poor HPV vaccine safety is
one of the main myths that must be dispelled by
communicating its safety profile, which has been
validated by decades of clinical trials and post-licensure studies involving tens of thousands of
participants.23 Additionally, concerns about serious
side-effects could be a response that conceals deeper
underlying reasons (eg, religious, societal, and
psychological issues). These reasons are potentially
culture-specific; their exploration may require
other forms of research rather than a questionnaire
approach (eg, focus group interviews).24
The second major barrier identified in this
study, ‘child too young’, is consistent with the
observation that most parents did not expect their
children to begin dating or engaging in sexual
activity until the age of 16 years or older. However,
in a survey of adolescents concerning sexual health
and their first sexual encounter, 1% of respondents
reported having sex for the first time at the age of
11 years, and 10% reported having sex at or before
the age of 15 to 16 years.25 Dating experience among
secondary school children in Hong Kong has
generally been consistent during the past 20 years,
such that approximately 30% of 12- to 14-year-olds
and 60% of 14- to 18-year-olds reported dating.26
Removal of the barrier ‘child too young’
requires providing parents with information
regarding the norms and realities of sexual behaviour
and encounters among teenagers in Hong Kong.
Additionally, parents must receive information
concerning the high prevalence of HPV infection
worldwide and in Hong Kong,27 as well as the high
rate of HPV transmission via skin-to-skin and
skin-to-mucosa contact during oral sex and non-penetrative
genital contact.28
In addition to considering the potential for
earlier-than-expected initiation of sexual activity,
parents should recognise that there is a biological
reason to vaccinate earlier. Data from clinical trials
show that HPV antibody titres are both higher and
more persistent among individuals who undergo
vaccination at a younger age.23 29 30 31
Cost of vaccination
At the time of this study, HPV vaccination was
unavailable to boys under the no-cost CIP;
awareness of HPV vaccination was lower in parents
of boys (68.5%), and HPV vaccine uptake in boys
was 27.1%. The current market price for two doses
of the HPV vaccine in Hong Kong is approximately
HK$3000 to HK$5000; this could be a prohibitive
cost for some families. Cost has been identified
as a key factor in many studies.32 Our findings
indicate that, if the government provided no-cost
HPV vaccination for boys, an additional 30% of
the parents of boys would agree to vaccination. In
addition to provision through a no-cost programme,
incorporation of the HPV vaccine into the CIP may
enhance parental confidence.33 The present findings
suggest that parents who spend more on healthcare
are less likely to accept HPV vaccination, indicating that preventive medicine is not a high priority
for these families. This hypothesis merits further
investigation; if confirmed, it must be addressed
through public health measures.
Gender-neutral vaccination
We recommend the adoption of a gender-neutral
HPV vaccination programme in Hong Kong. The
government should fully support and implement
such a programme for strong scientific and public
health reasons as outlined above. Furthermore, a
gender-neutral vaccination programme can achieve
the goal of HPV eradication with a lower coverage
rate of 55% to 70%, rather than the 80% to 90%
coverage required when only girls are vaccinated.34
In many advanced countries (eg, the United States,
Germany, and France), HPV vaccination coverage
rates remain low (20%-40%).34 Therefore, girls-only
vaccination programmes are unlikely to eliminate
HPV-related diseases. A gender-neutral vaccination
strategy must be universally implemented.
Limitations
There were some limitations in this study. First,
although the questionnaire was designed by
researchers with experience in surveys, HPV
infection and vaccination, it was not validated in
other studies. Nevertheless, the questionnaire was
pilot tested before launch, and it was both context-specific
and met the objectives of this study.
Second, the potential influence of healthcare
providers was not assessed in this study, although
previous studies have identified physician
recommendations as key predictors of HPV
vaccine uptake.35 36 37 38 However, the provision of HPV
vaccination through schools does not allow extensive
discussion with physicians; alternative opportunities
to engage healthcare providers must be explored.
Third, this study only targeted parents of
children in Primary 5 to 6; thus, no information was
available regarding HPV vaccine uptake in older
teenagers. It may have been useful to distinguish
between responses from mothers and fathers in this
study, considering the finding by Waller et al38 that,
compared with fathers, mothers in England and
Wales were more likely to agree to vaccinate.
Conclusion
The present findings suggest that raising awareness
of HPV vaccination, particularly among parents
of boys, is essential to increase the rate of vaccine
uptake. The provision of no-cost, school-based,
gender-neutral HPV vaccination through the CIP
would serve as a major boost to vaccine uptake.
When HPV vaccination is provided via schools
rather than healthcare clinics, clear and accessible
information must be provided to parents because they are the key decision makers in this situation.
The present findings suggest that parents need
more information about vaccine safety to alleviate
their concerns regarding serious side-effects. In the
future, the differences in uptake between the HPV
vaccine and other vaccines within the CIP may be
eliminated.
Author contributions
Concept or design: HYS Ngan, PKS Chan.
Acquisition of data: KY Kun, DFH Li, WY Wan.
Analysis or interpretation of data: EWH Lam.
Drafting of the manuscript: EWH Lam, PKS Chan.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: KY Kun, DFH Li, WY Wan.
Analysis or interpretation of data: EWH Lam.
Drafting of the manuscript: EWH Lam, PKS Chan.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
PKS Chan received honorarium and benefits in kind from
human papillomavirus vaccine manufacturers including
Merck Sharp & Dohme and GlaxoSmithKline as consultant,
speaker, and study investigator.
Acknowledgement
The authors thank members of the HPV Prevention Alliance, Hong Kong other than those listed in the authorship for their
support in this research, which was part of the activities
approved by the Alliance.
Declaration
Part of the content has been presented in a press conference organised by the HPV Prevention Alliance in Hong Kong on 13 May 2021.
Funding/support
This research was supported by a research grant from Merck Sharp & Dohme (Asia) Limited. The funder had no role in
study design, data collection, analysis, interpretation, or
manuscript preparation.
Ethics approval
The research was endorsed by the ethics panel of the HPV Prevention Alliance, which has given due consideration to the
ethical aspect of the study in the approval process.
References
1. World Health Organization. Cervical cancer. 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. Accessed 25 Nov 2021.
2. Brouwer AF, Campredon LP, Walline HM, et al. Incidence and clearance of oral and cervicogenital HPV infection: longitudinal analysis of the MHOC cohort study. BMJ Open 2022;12:e056502. Crossref
3. Kreimer AR, Pierce Campbell CM, Lin HY, et al. Incidence and clearance of oral human papillomavirus infection in
men: the HIM cohort study. Lancet 2013;382:877-87. Crossref
4. Wong MC, Vlantis AC, Liang M, et al. Persistence and clearance of oral human papillomavirus infections: a prospective population-based cohort study. J Med Virol 2020;92:3807-14. Crossref
5. International Agency for Research on Cancer. IARC Monographs on The Evaluation of Carcinogenic Risks to Humans. Volume 90: Human Papillomaviruses. Lyon, France: International Agency for Research on Cancer, World Health Organization; 2007.
6. Shiels MS, Kreimer AR, Coghill AE, Darragh TM, Devesa SS. Anal cancer incidence in the United States, 1977-2011: distinct patterns by histology and behavior. Cancer Epidemiol Biomarkers Prev 2015;24:1548-56. Crossref
7. Centers for Disease Control and Prevention, United States Government. Human Papillomavirus (HPV) vaccine: safety
information. 9 Sep 2020. Available from: https://www.cdc.gov/vaccinesafety/vaccines/hpv-vaccine.html. Accessed 7 Jun 2022.
8. Choi SE, Choudhary A, Huang J, Sonis S, Giuliano AR, Villa A. Increasing HPV vaccination coverage to prevent
oropharyngeal cancer: a cost-effectiveness analysis.
Tumour Virus Res 2022;13:200234. Crossref
9. Drolet M, Bénard É, Boily MC, et al. Population-level impact and herd effects following human papillomavirus
vaccination programmes: a systematic review and meta-analysis.
Lancet Infect Dis 2015;15:565-80. Crossref
10. Kudo R, Yamaguchi M, Sekine M, et al. Bivalent human papillomavirus vaccine effectiveness in a Japanese population: high vaccine-type-specific effectiveness and evidence of cross-protection. J Infect Dis 2019;219:382-90. Crossref
11. Hong Kong Cancer Registry, Hospital Authority. Cervical cancer in 2020. Available from: https://www3.ha.org.hk/cancereg/pdf/factsheet/2020/cx_2020.pdf. Accessed 6 Mar 2023.
12. Wu J. Cervical cancer prevention through cytologic and human papillomavirus DNA screening in Hong Kong Chinese women. Hong Kong Med J 2011;17(3 Suppl 3):20-4.
13. Hong Kong SAR Government. Community Care Fund to roll out pilot scheme on subsidised cervical cancer
screening and preventive education for eligible low-income
women. 12 Dec 2017. Available from: https://www.info.gov.hk/gia/general/201712/12/P2017121200366.htm. Accessed 25 Nov 2021.
14. Choi HC, Leung GM, Woo PP, Jit M, Wu JT. Acceptability and uptake of female adolescent HPV vaccination in Hong Kong: a survey of mothers and adolescents. Vaccine 2013;32:78-84. Crossref
15. Lee A, Ho M, Cheung CK, Keung VM. Factors influencing adolescent girls’ decision in initiation for human papillomavirus vaccination: a cross-sectional study in Hong Kong. BMC Public Health 2014;14:925. Crossref
16. Li SL, Lau YL, Lam TH, Yip PS, Fan SY, Ip P. HPV vaccination in Hong Kong: uptake and reasons for non-vaccination
amongst Chinese adolescent girls. Vaccine 2013;31:5785-8. Crossref
17. Chen JM, Leung DY. Factors associated with human papillomavirus vaccination among Chinese female university students in Hong Kong. Am Int J Soc Sci 2014;3:56-62.
18. Yuen WW, Lee A, Chan PK, Tran L, Sayko E. Uptake of human papillomavirus (HPV) vaccination in Hong Kong: facilitators and barriers among adolescent girls and their parents. PLoS One 2018;13:e0194159. Crossref
19. Cheung TH, Cheng S, Hsu D, et al. Public health and economic impact of gender-neutral nonavalent vaccination
and catch-up vaccination in Hong Kong. Poster presented
at: 37th Annual Meeting of the European Society for
Paediatric Infectious Diseases; 2019 May 6-11; Ljubljana, Slovenia.
20. Kmietowicz Z. Boys in England to get HPV vaccine from next year. BMJ 2018;362:k3237. Crossref
21. Takla A, Wiese-Posselt M, Harder T, et al. Background paper for the recommendation of HPV vaccination for boys in Germany. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2018;61:1170-86. Crossref
22. Chan D. Immunisation coverage for children aged two to five: findings of the 2015 immunisation survey. 2018.
Available from: https://www.chp.gov.hk/files/pdf/cdw_compendium_2017_revised.pdf. Accessed 21 Jun 2022.
23. Bednarczyk RA. Addressing HPV vaccine myths: practical information for healthcare providers. Hum Vaccin Immunother 2019;15:1628-38. Crossref
24. Siu JY, Lee A, Chan PK. Schoolteachers’ experiences of implementing school-based vaccination programs against human papillomavirus in a Chinese community: a qualitative study. BMC Public Health 2019;19:1514. Crossref
25. Hong Kong AIDS Foundation. A survey on “First Sex and Sexual Health”. 2018. Available from: https://www.aids.org.hk/?p=8856. Accessed 25 Nov 2021.
26. The Family Planning Association of Hong Kong. Report on Youth Sexuality Study 2016. 2017. Available from: https://www.famplan.org.hk/en/media-centre/press-releases/detail/fpahk-report-on-youth-sexuality-study. Accessed 25 Nov 2021.
27. Chan PK, Chang AR, Cheung JL, et al. Determinants of cervical human papillomavirus infection: differences between high- and low-oncogenic risk types. J Infect Dis 2002;185:28-35. Crossref
28. Petca A, Borislavschi A, Zvanca ME, Petca RC, Sandru F, Dumitrascu MC. Non-sexual HPV transmission and role of vaccination for a better future (review). Exp Ther Med 2020;20:186. Crossref
29. Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013;309:1793-802. Crossref
30. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014;63:1-30.
31. Romanowski B, Schwarz TF, Ferguson L, et al. Sustained immunogenicity of the HPV-16/18 AS04-adjuvanted
vaccine administered as a two-dose schedule in adolescent
girls: five-year clinical data and modeling predictions from
a randomized study. Hum Vaccin Immunother 2016;12:20-9. Crossref
32. Lee A, Wong MC, Chan TT, Chan PK. A home-school-doctor model to break the barriers for uptake of human
papillomavirus vaccine. BMC Public Health 2015;15:935. Crossref
33. Leask J, Chapman S, Hawe P, Burgess M. What maintains parental support for vaccination when challenged by anti-vaccination messages? A qualitative study. Vaccine 2006;24:7238-45. Crossref
34. Lehtinen M, Baussano I, Paavonen J, Vänskä S, Dillner J. Eradication of human papillomavirus and elimination of HPV-related diseases—scientific basis for global public health policies. Expert Rev Vaccines 2019;18:153-60.Crossref
35. Gamble HL, Klosky JL, Parra GR, Randolph ME. Factors influencing familial decision-making regarding human papillomavirus vaccination. J Pediatr Psychol 2010;35:704-15. Crossref
36. Radisic G, Chapman J, Flight I, Wilson C. Factors associated with parents’ attitudes to the HPV vaccination of their adolescent sons: a systematic review. Prev Med 2017;95:26-37. Crossref
37. Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage
among adolescents aged 13-17 years—United States, 2018.
MMWR Morb Mortal Wkly Rep 2019;68:718-23. Crossref
38. Waller J, Forster A, Ryan M, Richards R, Bedford H, Marlow L. Decision-making about HPV vaccination in parents of boys and girls: a population-based survey in England and Wales. Vaccine 2020;38:1040-7. Crossref