© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE CME
Best practices in epidermal growth factor
receptor T790M testing for advanced non–small-cell lung cancer in Hong Kong
Jonathan SF Nyaw, MB, ChB, FRCR1; KM Cheung, MB, ChB, MSc2; F Hioe, FRCPA, FKCPath3; Michael TY Kam, MB, ChB, FRCR4; Johnny KS Lau, FHKCR, FRCR5; YM Lau, MRCP, FHKCP6; Dennis KC Leung, FHKCR, FHKAM (Radiology)5; Fiona MY Lim, MB, BS, FRCR7
1 Department of Clinical Oncology, Tuen Mun Hospital, Hong Kong SAR, China
2 Department of Clinical Oncology, Queen Elizabeth Hospital, Hong Kong SAR, China
3 Department of Pathology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
4 Department of Clinical Oncology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
5 Department of Clinical Oncology, Queen Mary Hospital, Hong Kong SAR, China
6 Department of Clinical Oncology, Prince of Wales Hospital, Hong Kong SAR, China
7 Department of Oncology, Princess Margaret Hospital, Hong Kong SAR, China
Corresponding author: Dr Jonathan SF Nyaw (sfnyaw@ha.org.hk)
Abstract
The T790M mutation in the epidermal growth factor
receptor gene causes most acquired resistance to firstor
second-line epidermal growth factor receptor–tyrosine kinase inhibitors in advanced non–small-cell
lung cancer. The results of T790M testing can
guide subsequent treatment. Despite the availability
of guidelines from international organisations,
T790M testing practices in Hong Kong must be
streamlined and adapted to the Hospital Authority
setting. To address this issue, a panel of experts in
oncology and pathology met for discussion of key
topics regarding T790M testing practices in Hong
Kong, including the appropriate timing of testing
and re-testing, as well as optimal testing methods.
All panel members voted on the results of the
discussion to achieve consensus. Items supported by
a majority vote were adopted as consensus statements
regarding current best practices for T790M testing in
Hong Kong. Among the topics discussed, the panel
agreed that T790M testing should be initiated upon
radiological progression, including symptomatic
disease progression or central nervous system–only
progression. The experts also preferred initial testing
with liquid biopsy, using the widely available digital
polymerase chain reaction platform. This document
provides the final consensus statements, as well as a testing and treatment workflow, for clinicians in
Hong Kong to use as guidance in T790M testing.
Introduction
Epidermal growth factor receptor (EGFR)–directed
tyrosine kinase inhibitors (TKIs) are recommended
as first-line therapy for non–small-cell lung cancer
(NSCLC) carrying a sensitising mutation in the EGFR
gene. Compared with platinum-based chemotherapy,
first- and second-generation EGFR-TKIs have shown
superior efficacy; they are regarded as the standard
of care for advanced NSCLC.1 2 However, acquired
resistance to EGFR-TKIs eventually occurs, leading
to disease progression.3 The T790M substitution
mutation in exon 20, present in 50% to 60% of cases,
is the most frequent cause of resistance to first- and
second-generation EGFR-TKIs.4 5 6 According to a
laboratory report in Hong Kong (Sanomics, unpublished data presented in a meeting on 27 June 2019), the
proportion of T790M-positive NSCLC cases across
seven hospitals under the Hospital Authority from 2017 to 2020 (n=3398) ranged from 19.0% to 32.8%.
In cases of resistance to first- and second-generation TKIs, next-line therapeutic options were
limited prior to the introduction of osimertinib, a
TKI selective for EGFR-sensitising mutations and the
T790M resistance mutation. Osimertinib received
accelerated Food and Drug Administration approval
in the United States in November 2015, along with a
companion diagnostic test for the T790M mutation;
it received full approval in March 2017 based on the
results of the AURA3 study.7 The AURA trials were
included in a clinical investigation of osimertinib as
second-line therapy in T790M-positive NSCLC.7
AURA3 was a randomised, open-label phase 3 trial
that enrolled patients with T790M-positive advanced
NSCLC refractory to first-generation TKIs (n=419).
The study showed that progression-free survival
was significantly longer and the response rate was significantly higher in patients receiving osimertinib
than in patients receiving platinum-pemetrexed
chemotherapy.8 Osimertinib is now regarded as the standard of care for patients with T790M-positive
tumours and acquired TKI resistance.1 2 9
Molecular analysis of T790M mutation status
should be performed upon progression of EGFR-mutated
NSCLC to identify patients for whom
osimertinib would be an appropriate next-line
option. The DNA used for testing can be obtained
via repeated biopsy of tissue, or by analysis of circulating tumour DNA (ctDNA) circulating
in blood or other body fluids (ie, liquid biopsy).10
Technologies available for T790M detection in tissue
and body fluids include real-time polymerase chain
reaction (PCR)–based methods such as the Cobas
and Therascreen tests, and digital PCR (dPCR)
platforms such as droplet digital PCR (ddPCR)
and BEAMing (beads, emulsions, amplification,
magnetics).11 Next-generation sequencing (NGS)
is a high-throughput sequencing method that can
simultaneously analyse variable regions of the
genome and detect somatic mutations (eg, single-nucleotide
variations, copy number variations, and
insertion/deletions or gene fusions); the method can
also be used to detect the T790M mutation as well
as other genomic alterations that cause EGFR-TKI
resistance.9
Guidelines published in the past several years
have outlined recommendations for T790M testing
within the context of a region’s reimbursement policy,
hospital system, and laboratory infrastructure.12 13 To
provide guidance to local hospitals under the Hong
Kong Hospital Authority, a panel of Hong Kong
experts was convened to discuss current practices
in T790M testing and adaptations to promote
optimal patient outcomes. This report summarises
the resulting consensus statements, while proposing
an algorithm for T790M testing and subsequent
NSCLC treatment, which is intended to serve as a
guidance for clinicians regarding best practices in
EGFR T790M testing.
Methods
A panel of seven oncologists and a pathologist was convened to participate in the development of a
consensus document regarding best practices in
T790M testing in Hong Kong. During the initial
face-to-face meeting, the panel members reviewed
current EGFR T790M testing practices in Hong
Kong Hospital Authority hospitals, then discussed
relevant evidence and practical considerations. After
the identification of knowledge gaps and differences
in T790M testing practices within Hong Kong, the
panel proposed key questions regarding the timing
and procedures of testing, along with relevant
clinical scenarios.
A second meeting was convened to discuss the best practices for T790M testing, in response
to the key questions previously drafted. After
each member’s queries and comments had been
considered by the panel, the members indicated their
agreement with, or selection among, the responses
presented (online supplementary Appendix). If the
majority of the panel agreed with a response, it was
regarded as a current best practice and adopted
as a consensus statement. Individual members’
comments based on practical experience in the
field were integrated with the chosen responses to
formulate the final consensus statements.
Recommendations
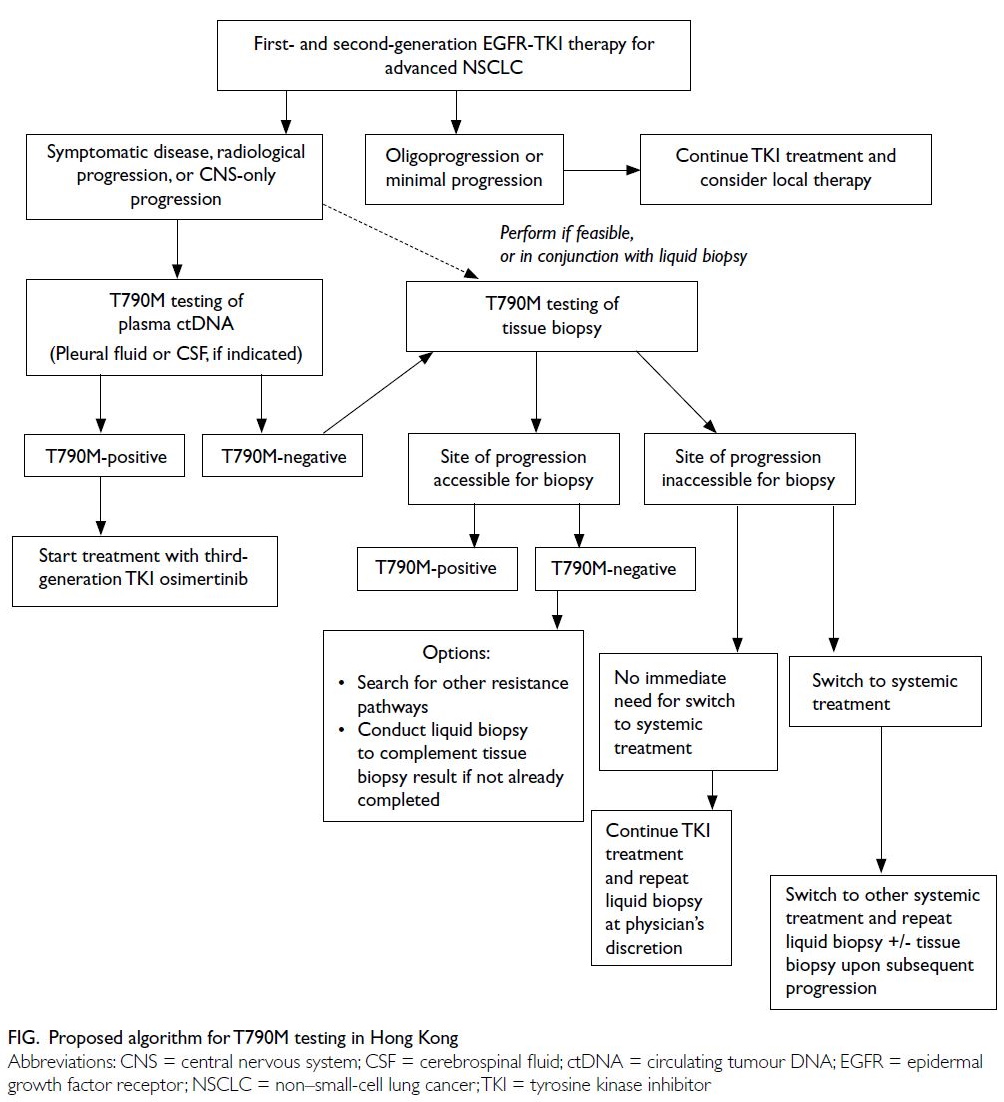
When to test for the EGFR T790M mutation
The panel members unanimously agreed that
patients with EGFR-mutated NSCLC treated with
first- or second-generation TKIs should undergo
T790M testing upon radiological disease progression
(eg, asymptomatic progression, symptomatic disease
progression, or central nervous system [CNS]–only
progression) [Table 1 and Fig]. Testing is warranted
because these events indicate progression that may
require modified treatment. Although the underlying
premise of T790M testing involves assessing
eligibility for third-line TKI inhibition, clinicians
should concurrently investigate the feasibility of
local therapy for oligoprogressive disease.
Biochemical progression (eg, an increasing
carcinoembryonic antigen [CEA] level) may prompt
clinicians to perform additional investigation of
tumour status; however, biochemical progression
alone is insufficient to indicate a need for T790M
testing. Radiological progression is usually defined by the Response Evaluation Criteria in Solid Tumours,
which are typically used for objective assessment of
tumour burden in clinical trials.3 Testing is indicated
upon radiological progression, but panel members
acknowledged that the definition of radiological progression may differ among clinicians.
Patients with symptomatic disease progression
can experience rapid deterioration; thus, immediate
assessment of T790M mutation status is needed to
plan subsequent treatments that are likely to confer
benefit, such as osimertinib.14 15 Similarly, regardless
of the patient’s clinical state (symptomatic or
asymptomatic), immediate testing is indicated for
CNS-only progression because the condition carries
a poor prognosis. Patients with T790M-positive CNS
progression may also benefit from osimertinib, which
has compelling efficacy data with respect to CNS
metastases, including asymptomatic cases.16 17 18 In
the AURA3 trial, osimertinib showed superior CNS
efficacy compared with platinum- or pemetrexed-based chemotherapy; it also demonstrated activity
against leptomeningeal metastasis.16 18 In the phase
3 FLAURA study, osimertinib had superior CNS
efficacy compared with gefitinib or erlotinib.17
Oligoprogression (new lesions or regrowth in
a few areas)19 alone does not warrant T790M testing
and can be managed by local ablative therapy. Local
therapy may prolong disease control. For example,
two studies of patients with oligometastatic NSCLC
while on standard TKI therapy revealed a median
time to progression of 6.2 to 10.0 months from
the initiation of local therapy and continuation of
previous TKI.20 21
The panel members agreed that CEA level is
not a reliable marker of disease progression1 2; CEA
analysis alone should not be used to determine the
need for T790M testing. However, an elevated CEA
level suggests that disease progression should be
closely monitored by other investigation methods.
The level may be elevated in conjunction with
radiological progression; consideration of CEA level
and any evidence of radiological progression can help
clinicians to determine subsequent management.
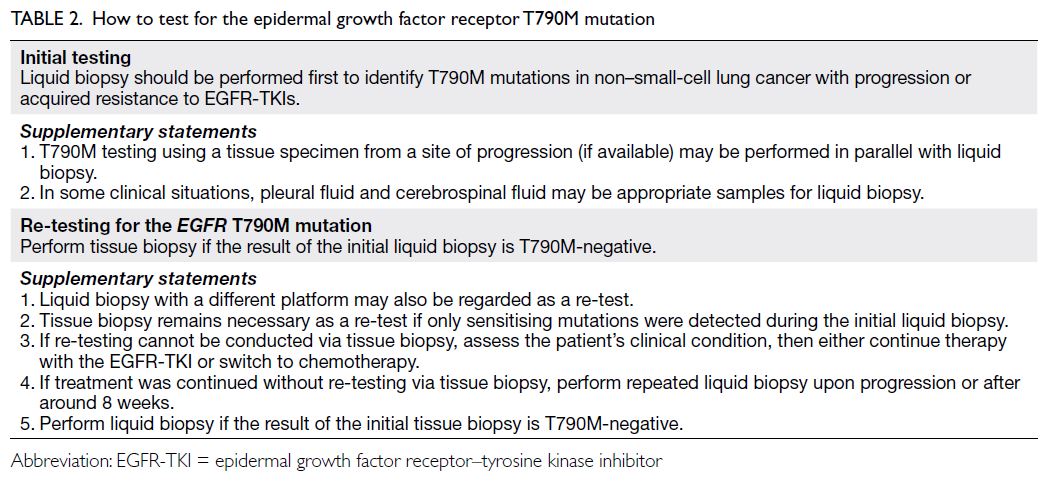
How to test for the EGFR T790M mutation
Initial testing
All panel members supported the use of liquid biopsy for initial T790M testing (Table 2). Liquid biopsy
was the preferred method because it allows non-invasive
assessment of tumour biology, is readily
available, and has a short turnaround time.9 10 22
Conditions that may support the use of liquid biopsy
as the first choice for T790M testing include limited
tumour tissue availability, low tissue sample quality,
poor patient health that precludes tissue biopsy,
and patient refusal of tissue biopsy.1 2 9 10 Published
guidelines from Australia, the United States National
Comprehensive Cancer Network, and the European Society for Medical Oncology also recommend
liquid biopsy for initial T790M testing.1 9 13
A liquid biopsy is generally conducted by
collecting plasma ctDNA. The detection of EGFR
mutations in plasma ctDNA has high concordance
with tissue-based detection (up to 74%).11 Analyses
of plasma ctDNA have high specificity but moderate
sensitivity; thus, negative plasma results should be
confirmed by tissue biopsy.9 10 13 23
Other biological fluids (eg, pleural fluids and
cerebrospinal fluid [CSF]) can be used to provide
ctDNA for liquid biopsy. The majority (88%) of
panel members would send pleural fluid (when
available) for liquid biopsy. The EGFR mutations can
be detected via ctDNA from pleural effusion fluid;
however, if a sufficient number of cells is collected,
cell block analysis may be an alternative diagnostic
method.24 25
The majority (80%) of panel members would
also request CSF-based liquid biopsy in the event of
CNS metastasis. Cerebrospinal fluid is suitable for
ctDNA analysis of tumour mutations in patients with
CNS metastasis or leptomeningeal metastasis.26 27 28
Although CSF sampling for T790M testing requires
the invasive lumbar puncture procedure, CSF is
considered an accessible representation of EGFR
mutation status in the brain and leptomeningeal
metastases, which are typically inaccessible;
therefore, CSF analysis is regarded as a useful adjunct
to plasma analysis.26 27
If a tissue sample is available, tissue sample–based T790M testing can be performed in parallel
with liquid biopsy–based testing. This approach
is supported by the Canadian guideline panel,
the International Association for the Study of
Lung Cancer, and a Pan-Asian group that adopted
the European Society for Medical Oncology
guidelines.2 10 12
The panel members agreed that all tissue
samples for T790M testing should be collected from
accessible and untreated sites of progression. Any
type of tissue is acceptable, except necrotic tissue.
Furthermore, if a bone lesion sample is used for
biopsy, it should have minimal decalcification to
ensure that DNA quality is sufficient for molecular
analysis.
Tissue biopsy–based analyses have some
limitations. For example, lung biopsy is an invasive
procedure with potential complications, such as
intrapulmonary haemorrhage and pneumothorax.29 30
Additionally, intratumour and intermetastatic
heterogeneity in biopsied tissue may lead to false-negative
results.31
Re-testing
The panel members agreed that re-testing should
be performed if the initial liquid biopsy is T790M-negative.
Considering that plasma liquid biopsy
has a false-negative rate of 30%, tissue biopsy is
warranted to confirm T790M mutation status if the
result of the initial plasma liquid biopsy is T790M-negative
(Table 2).23 Failure to detect the original
sensitising mutation via liquid biopsy may be related
to various factors, including suboptimal sample
preservation or a non-secretory tumour, and further
testing is highly recommended. If the initial liquid
biopsy was performed with a less sensitive assay
(eg, real-time PCR), a more sensitive assay such as
dPCR or NGS should be considered. If tissue biopsy
is indicated, it should be collected from a site of
progressive disease.
Confirmatory re-testing is intended to
guide clinicians in the selection of appropriate
therapy; although tissue biopsy is the preferred
re-test approach, factors such as site accessibility,
patient symptoms, and performance status should
be considered when determining re-test timing.
The following treatment options may be suitable
alternatives to early tissue repeated biopsy: continue
EGFR-TKI therapy and perform repeated liquid
biopsy later, or switch to chemotherapy and perform
repeated liquid biopsy upon progression.
The optimal timing for repeated liquid
biopsy is unknown. Most panel members (86%)
would perform repeated liquid biopsy if there was evidence of further progression, including
worsening symptoms. In contrast, for asymptomatic
patients or patients with slowly progressing disease
who continued to receive EGFR-TKI therapy,
panel members suggested a minimum of 8 weeks
between repeated liquid biopsies. In real-world
setting, 8 weeks is the typical interval for further
progression from the time that a patient continues
TKI therapy after the first progression event; further
progression at that time would suggest a need for
systemic treatment, rather than TKIs. Additionally,
in phase 2 studies, tumour assessments are typically
performed at around 8-week intervals to coincide
with the end of a treatment cycle.32 For example,
in the phase 2 ASPIRATION study that included
a cohort of patients with advanced NSCLC who
continued TKI therapy after progression, plasma
analysis was generally conducted every 8 weeks. The
study showed that the median time between the first
and the second progression events was approximately 3
months.33
Most panel members (88%) agreed that, when
tissue biopsy is used as the initial test, a T790M-negative
result should be confirmed by liquid
biopsy. Although the standard of care constitutes
tissue biopsy using an adequate sample from a site
of progression, tumour heterogeneity may lead to
a false-negative result. Subsequent liquid biopsy
using ctDNA may complement the T790M-negative
findings of initial tissue biopsy.
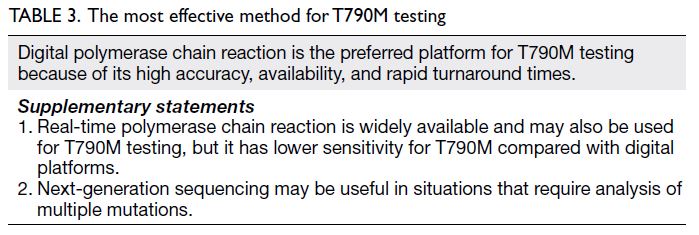
The most effective method for EGFR T790M
testing
In Hong Kong, plasma samples are generally tested
by validated targeted assays, such as real-time PCR,
ddPCR, or NGS. The assay used for liquid biopsy
depends on the hospital’s laboratory infrastructure,
but all assays should be able to detect T790M in ≤5%
of viable cells.9 10
All panel members expressed a preference for
dPCR to detect T790M via liquid biopsy (Table 3).
An important consideration is that dPCR platforms
have higher sensitivity than real-time PCR—ddPCR
has a sensitivity of approximately 80% or higher for
T790M.11 34 In patients who showed progression
while receiving EGFR-TKIs, ddPCR had a positivity
rate of 66% for T790M, whereas Cobas real-time
PCR had a positivity rate of 24%.35 Next-generation
sequencing has also shown high sensitivity for
T790M.11 22 34 Furthermore, NGS can be used to
analyse other genes implicated in the EGFR-TKI
resistance (eg, MET, BRAF, ERBB2 [HER2], and
KRAS), in conjunction with T790M testing.
Tissue samples can also be tested by real-time
PCR or NGS. In Hong Kong, real-time PCR is
commonly used for T790M testing. As mentioned
above, NGS has a high sensitivity for T790M34
and can provide additional genetic information regarding the mechanism of EGFR-TKI resistance.
For repeated liquid biopsy, ddPCR is the preferred
assay, but liquid-based NGS can also be considered.
Conclusion
Molecular profiling of T790M mutation status in
NSCLC with acquired resistance provides important
guidance for clinicians with respect to next-line
treatment. It can identify patients who are candidates
for second- or third-line treatment with osimertinib,
which has demonstrated superior efficacy, compared
with chemotherapy, in the management of advanced
NSCLC refractory to first- or second-generation
TKIs.10
In Hong Kong, liquid biopsy assessment by
a sensitive ctDNA platform is recommended as
the first-line option for T790M testing to facilitate
clinical decision making. Because of its accuracy and
availability, dPCR is the preferred platform for this
assessment. This expert panel developed consensus
statements (Tables 1, 2 and 3) and a corresponding
workflow for T790M testing (Fig). Clinicians in Hong
Kong can use the proposed workflow to guide the
T790M testing process from the initial step of liquid
biopsy to the determination of clinically appropriate
situations for re-testing, followed by selection of
treatment approaches.
In the future, T790M testing guidelines can be
refined by adding the experience of multidisciplinary
experts and new knowledge gained from research in
Hong Kong and other countries.
Author contributions
Concept or design: JSF Nyaw, F Hioe, MTY Kam, JKS Lau, FMY Lim.
Acquisition of data: JSF Nyaw, F Hioe, MTY Kam, YM Lau, FMY Lim.
Analysis or interpretation of data: JSF Nyaw, F Hioe, MTY Kam.
Drafting of the manuscript: JSF Nyaw, KM Cheung, F Hioe, MTY Kam.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: JSF Nyaw, F Hioe, MTY Kam, YM Lau, FMY Lim.
Analysis or interpretation of data: JSF Nyaw, F Hioe, MTY Kam.
Drafting of the manuscript: JSF Nyaw, KM Cheung, F Hioe, MTY Kam.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
We thank Dr Ben Searle and Dr Pia Villanueva of MIMS (Hong Kong) Limited for editorial support, which was funded
by AstraZeneca Hong Kong Limited (Hong Kong).
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
1. Planchard D, Popat S, Kerr K, et al. Metastatic non-small
cell lung cancer: ESMO Clinical Practice Guidelines
for diagnosis, treatment and follow-up. Ann Oncol
2018;29(Suppl 4):iv192-237. Crossref
2. Wu YL, Planchard D, Lu S, et al. Pan-Asian adapted Clinical
Practice Guidelines for the management of patients with
metastatic non-small-cell lung cancer: a CSCO-ESMO
initiative endorsed by JSMO, KSMO, MOS, SSO and TOS.
Ann Oncol 2019;30:171-210. Crossref
3. Jackman D, Pao W, Riely GJ, et al. Clinical definition of
acquired resistance to epidermal growth factor receptor
tyrosine kinase inhibitors in non-small-cell lung cancer. J
Clin Oncol 2010;28:357-60. Crossref
4. Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers
acquiring resistance to EGFR inhibitors. Sci Transl Med
2011;3:75ra26. Crossref
5. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI
therapy in 155 patients with EGFR-mutant lung cancers.
Clin Cancer Res 2013;19:2240-7. Crossref
6. Campo M, Gerber D, Gainor JF, et al. Acquired resistance to first-line afatinib and the challenges of prearranged progression biopsies. J Thorac Oncol 2016;11:2022-6. Crossref
7. United States Food and Drug Administration. Osimertinib (Tagrisso). Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/osimertinib-tagrisso. Accessed 2 Sep 2020.
8. Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017;376:629-40. Crossref
9. National Comprehensive Cancer Network. Non-small cell lung cancer. Version 4.2021. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed 14 May 2021.
10. Lindeman NI, Cagle PT, Aisner DL, et al. Updated
molecular testing guideline for the selection of lung
cancer patients for treatment with targeted tyrosine
kinase inhibitors: guideline from the College of American
Pathologists, the International Association for the Study of
Lung Cancer, and the Association for Molecular Pathology.
J Thorac Oncol 2018;13:323-58. Crossref
11. Thress KS, Brant R, Carr TH, et al. EGFR mutation
detection in ctDNA from NSCLC patient plasma: a cross-platform
comparison of leading technologies to support
the clinical development of AZD9291. Lung Cancer
2015;90:509-15. Crossref
12. Stockley T, Souza CA, Cheema PK, et al. Evidence-based best practices for EGFR T790M testing in lung cancer in
Canada. Curr Oncol 2018;25:163-9. Crossref
13. John T, Bowden JJ, Clarke S, et al. Australian recommendations for EGFR T790M testing in advanced
non-small cell lung cancer. Asia Pac J Clin Oncol
2017;13:296-303. Crossref
14. Yoshida T, Yoh K, Niho S, et al. RECIST progression patterns during EGFR tyrosine kinase inhibitor treatment of advanced non-small cell lung cancer patients harboring
an EGFR mutation. Lung Cancer 2015;90:477-83.Crossref
15. Yang JJ, Chen HJ, Yan HH, et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent
management in advanced non-small cell lung cancer. Lung
Cancer 2013;79:33-9. Crossref
16. Wu YL, Ahn MJ, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced
non-small-cell lung cancer: data from a randomized phase
III trial (AURA3). J Clin Oncol 2018;36:2702-9. Crossref
17. Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth
factor receptor tyrosine kinase inhibitors in patients with
untreated EGFR-mutated advanced non-small-cell lung
cancer. J Clin Oncol 2018;36:3290-7. Crossref
18. Ahn MJ, Chiu CH, Cheng Y, et al. Osimertinib for patients with leptomeningeal metastases associated with
EGFR T790M-positive advanced NSCLC: the AURA
leptomeningeal metastases analysis. J Thorac Oncol
2020;15:637-48. Crossref
19. Gandara DR, Li T, Lara PN, et al. Acquired resistance to targeted therapies against oncogene-driven non-small-cell lung cancer: approach to subtyping progressive disease and
clinical implications. Clin Lung Cancer 2014;15:1-6. Crossref
20. Yu HA, Sima CS, Huang J, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a
treatment strategy in EGFR-mutant advanced lung cancers
that have developed acquired resistance to EGFR tyrosine
kinase inhibitors. J Thorac Oncol 2013;8:346-51. Crossref
21. Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative
therapy of oligoprogressive disease prolongs disease
control by tyrosine kinase inhibitors in oncogene-addicted
non-small-cell lung cancer. J Thorac Oncol 2012;7:1807-14. Crossref
22. Liang Z, Cheng Y, Chen Y, et al. EGFR T790M ctDNA testing platforms and their role as companion diagnostics: correlation with clinical outcomes to EGFR-TKIs. Cancer
Lett 2017;403:186-94. Crossref
23. Oxnard GR, Thress KS, Alden RS, et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol 2016;34:3375-82. Crossref
24. Yang J, Lee OJ, Son SM, et al. EGFR mutation status in lung adenocarcinoma-associated malignant pleural effusion and efficacy of EGFR tyrosine kinase inhibitors. Cancer Res Treat 2018;50:908-16. Crossref
25. Kimura H, Fujiwara Y, Sone T, et al. EGFR mutation status in tumour-derived DNA from pleural effusion fluid is a practical basis for predicting the response to gefitinib. Br J
Cancer 2006;95:1390-5. Crossref
26. Huang R, Xu X, Li D, et al. Digital PCR-based detection of EGFR mutations in paired plasma and CSF samples of
lung adenocarcinoma patients with central nervous system
metastases. Target Oncol 2019;14:343-50. Crossref
27. Li YS, Jiang BY, Yang JJ, et al. Unique genetic profiles
from cerebrospinal fluid cell-free DNA in leptomeningeal
metastases of EGFR-mutant non-small-cell lung cancer: a
new medium of liquid biopsy. Ann Oncol 2018;29:945-52. Crossref
28. Pan W, Gu W, Nagpal S, Gephart MH, Quake SR. Brain tumor mutations detected in cerebral spinal fluid. Clin
Chem 2015;61:514-22. Crossref
29. Chouaid C, Dujon C, Do P, et al. Feasibility and clinical impact of re-biopsy in advanced non small-cell lung cancer: a prospective multicenter study in a real-world setting (GFPC study 12-01). Lung Cancer 2014;86:170-3. Crossref
30. Yoon HJ, Lee HY, Lee KS, et al. Repeat biopsy for mutational analysis of non-small cell lung cancers resistant to previous chemotherapy: adequacy and complications. Radiology 2012;265:939-48. Crossref
31. Bedard PL, Hansen AR, Ratain MJ, Siu LL. Tumour heterogeneity in the clinic. Nature 2013;501:355-64. Crossref
32. Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur
J Cancer 2016;62:132-7. Crossref
33. Park K, Yu CJ, Kim SW, et al. First-line erlotinib therapy until and beyond Response Evaluation Criteria in Solid Tumors progression in Asian patients with epidermal
growth factor receptor mutation-positive non-small-cell
lung cancer: the ASPIRATION study. JAMA Oncol
2016;2:305-12. Crossref
34. Passiglia F, Rizzo S, Di Maio M, et al. The diagnostic accuracy of circulating tumor DNA for the detection of EGFR-T790M mutation in NSCLC: a systematic review and meta-analysis. Sci Rep 2018;8:13379. Crossref
35. Buder A, Setinek U, Hochmair MJ, et al. EGFR mutations in cell-free plasma DNA from patients with advanced lung adenocarcinoma: improved detection by droplet digital
PCR. Target Oncol 2019;14:197-203. Crossref