Hong Kong Med J 2023 Jun;29(3):224–32 | Epub 12 Jun 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Descriptive analysis of poisoning cases involving
attention deficit hyperactivity disorder medications in Hong Kong
L Gao, MSc1; Kenneth KC Man, PhD1,2; ML Tse, MB, ChB3; Anthony TY Chow, MB, BS3; Kirstie HTW Wong, BSc4; Esther W Chan, PhD1; Celine SL Chui, PhD5,6; David Coghill, MD7; KL Hon, MB, BS, MD8; Patrick Ip, MB, BS4; Ian CK Wong, PhD1,2
1 Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
2 Centre for Medicines Optimisation Research and Education, Research Department of Policy and Practice, University College London School of Pharmacy, London, United Kingdom
3 Hong Kong Poison Information Centre, United Christian Hospital, Hong Kong SAR, China
4 Department of Paediatrics and Adolescent Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
5 School of Nursing, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
6 School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
7 Department of Paediatrics and Psychiatry, Faculty of Medicine, Dentistry and Health Sciences, University of Melbourne, Melbourne, Australia
8 Department of Paediatrics, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
Corresponding author: Prof Ian CK Wong (wongick@hku.hk)
Abstract
Introduction: The number of poisoning cases involving attention deficit hyperactivity disorder
(ADHD) medications has reportedly risen with their
increased use. However, there is limited relevant
evidence from Asia. We analysed the characteristics
of poisoning events involving these medications in
Hong Kong.
Methods: We retrieved data regarding ADHD
medication–related poisoning cases from the Hong
Kong Poison Information Centre and conducted a
descriptive analysis of the demographic information
and poisoning information including sources of cases,
exposure reason, exposure location, and outcome.
The HKPIC data were linked with the Hospital
Authority Clinical Data Analysis and Reporting
System (CDARS) via de-identified Accident and
Emergency numbers of public hospitals to investigate
clinical characteristics. We also retrieved ADHD
medication prescription records from the CDARS,
then compared trends between poisoning cases and
ADHD medication use.
Results: We identified 72 poisoning cases involving
ADHD medications between 2009 and 2019, of which
approximately 70% occurred in the affected individual’s
residence; most were intentional poisoning events
(65.3%). No statistically significant association was
observed between ADHD medication prescription
trends and poisoning events involving ADHD
medications. Of the 66 cases (91.7%) successfully
linked to CDARS, 40 (60.6%) occurred in individuals
with ADHD (median age: 14 years); 26 (39.4%)
occurred in individuals who lacked ADHD (median
age: 33 years) but displayed higher rates of other
mental disorders including depression and anxiety.
Conclusion: No significant correlation was evident between ADHD medication prescriptions and
poisoning events involving ADHD medications.
However, medication management and caregiver
education must be emphasised to prevent potential
poisoning events.
New knowledge added by this study
- The number of prescriptions for attention deficit hyperactivity disorder (ADHD) medications increased by 2.6-fold between 2009 and 2019 (from 32 497 to 84 037).
- In total, 72 poisoning cases involving ADHD medications between 2009 to 2019 were confirmed by the Hong Kong Poison Information Centre, and there was no clear trend regarding the annual number of such cases.
- No statistically significant correlation was evident between ADHD medication prescriptions and poisoning events involving ADHD medications.
- The management and safe storage of ADHD medications should be strengthened in both ADHD and non-ADHD populations.
- Appropriate interventions and/or social support for individuals with psychiatric disorders should be planned and implemented to reduce the risk of poisoning.
Introduction
Acute poisoning by medicines or chemicals is
common worldwide; it can lead to death and other
serious outcomes. Globally, poison centres are
estimated to receive millions of calls each year
regarding acute poisoning reports or consultations.1
According to the Hong Kong Poison Information
Centre (HKPIC) annual reports from 2009 to 2018,
>3000 events of poisoning in Hong Kong each year
are potentially caused by medications or chemicals
(excluding food poisoning and bites or stings).2 3 4 5 6 7 8 9 10 11
Attention deficit hyperactivity disorder
(ADHD) is the most prevalent neurodevelopmental
disorder in childhood and adolescence.12 13 Because
of its inattentiveness, hyperactivity, and impulsivity
characteristics, individuals with ADHD have higher
risks of intentional and accidental poisoning.14 A
recent study showed that ADHD medication use
increased in many countries and regions from 2001
to 2015, including the United States (US), the United
Kingdom, Australia, and Hong Kong.15 Descriptive
analyses of poisoning cases reported to the US16 and
Australian poison centres17 showed trends similar to the reported increases in ADHD medication
prescriptions. Accordingly, we hypothesised that an increase in ADHD medication prescriptions would lead to an increase in the number of poisoning cases involving ADHD medications in Hong Kong.
To our knowledge, there have been no relevant
studies regarding trends in poisoning cases involving
ADHD medications in Hong Kong; it is unclear
whether the increased use of ADHD medications is
associated with an increased risk of overall poisoning
in Hong Kong. Therefore, this study analysed the
trends and characteristics of poisoning events
involving ADHD medications in Hong Kong.
Methods
Participants and databases
The data used in this study include HKPIC poisoning
records for the period between 1 January 2009 and
31 December 2019, consisting of consultations
(poisoning cases in which healthcare professionals
consulted the HKPIC for poison information and
management advice) and poisoning cases reported
to the accident and emergency (A<E) departments
under Hospital Authority (HA).11
We used A<E numbers, which are de-identified
codes generated by the HA, to link data
from the HKPIC with poisoning data acquired from
the A<E module of the Clinical Data Analysis and
Reporting System (CDARS) of the HA. The CDARS
is an electronic health records system that contains
patient demographic and clinical information
from inpatient, outpatient, and A<E settings. It
captures data from all public hospitals and clinics
in Hong Kong18 19 20 and has been extensively used
for safety studies regarding ADHD and ADHD
medications.21 22 23 24 25 Reference keys (ie, de-anonymised
identifiers in the CDARS) were used for matched
individuals to retrieve relevant diagnostic and
prescription information. The data presented are
fully anonymised, and the risk of identification is
minimal.
Statistical analysis
The annual prevalence of poisoning cases involving ADHD medications was calculated, along with
the annual prevalence of ADHD medication
prescriptions dispensed by the HA; the relationship
between the two prevalence trends was examined
using a cross-correlation function.26 Demographic
and clinical details were summarised to include
the exposure reason, exposure location, and
clinical outcome of each case. Definitions and
classifications of clinical outcomes were acquired
from the HKPIC (Table 1).11 Subgroup analyses
were conducted to examine the association between
the annual prevalence of poisoning related to
ADHD medications in individuals with ADHD (ie,
individuals with an ADHD diagnosis or a prescription
for ADHD medication), and the annual prevalence of prescriptions dispensed by the HA. These analyses
were conducted using information from the CDARS,
including prescriptions and diagnoses. R software
(version 4.0.3) and Microsoft Excel 2019 were used
for analyses.
This descriptive analysis has been reported in
accordance with the STROBE (Strengthening the
Reporting of Observational Studies in Epidemiology)
checklist for cross-sectional studies.
Results
In total, 72 poisoning cases involving ADHD
medications were confirmed by the HKPIC during
the period from 2009 to 2019. The trends in all
poisoning cases involving ADHD medications
and the annual number of ADHD medication
prescriptions are shown in Figure 1. The number
of ADHD medication prescriptions is increasing
annually, from 32 497 in 2009 to 84 037 in 2019; in
contrast, although the number of poisoning cases
involving ADHD medications has fluctuated, there
has been no upward trend. There were similar findings
with respect to poisoning cases involving ADHD
medications in patients with an ADHD diagnosis
or prescription (Fig 1). Notably, from 2014 to 2015,
when the rate of increase in ADHD medication
prescriptions was faster than in the previous year,
the number of poisoning cases involving ADHD
medications showed an upward trend. In contrast,
when the rate of increase in ADHD medication
prescriptions was slower in the previous year, the
number of poisoning cases decreased (eg, from
2015 to 2016). However, cross-correlation analysis
did not reveal any significant correlation between
the number of poisoning cases and the number of
ADHD medication prescriptions. At a time lag of 0,
the correlations were approximately 0.442 and 0.326
for all poisoning cases involving ADHD medications and poisoning cases involving ADHD medications
in patients with ADHD, respectively; these values
were within the range of -0.59 to 0.59, indicating the
absence of a significant correlation. At a time lag of
1 (ie, 1 year), the correlations were 0.176 and 0.063,
respectively; neither correlation was statistically significant.
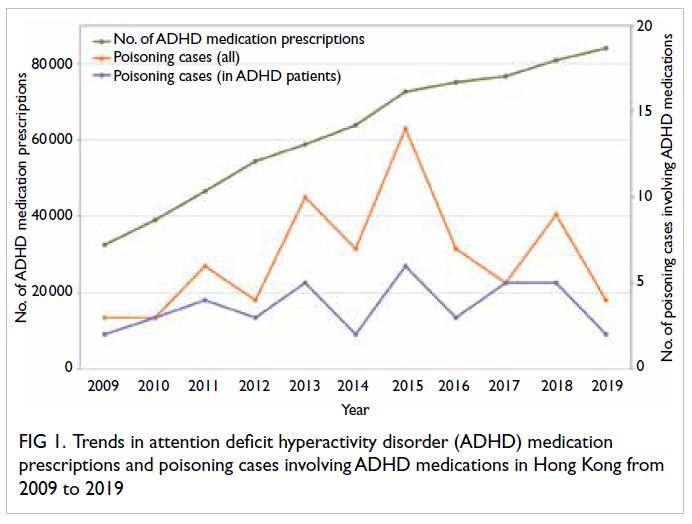
Figure 1. Trends in attention deficit hyperactivity disorder (ADHD) medication prescriptions and poisoning cases involving ADHD medications in Hong Kong from 2009 to 2019
Among the 72 poisoning cases involving
112 substances, most (n=56; 77.8%) were caused
by a single ADHD medication (Fig 2). The 112
substances used in the poisoning cases are shown
in Figure 3. Each case included at least one type of ADHD medication, the most common of which
was methylphenidate (n=70; 95.9%). Furthermore,
17% of the substances that caused poisoning were
psychotropic drugs.
The main characteristics of all poisoning cases
involving ADHD medications are shown in Table 2.
The two main sources of these poisoning cases
were consultation (54.2%) and reporting (40.3%);
most exposures occurred in the affected individual’s
residence. The exposure reason was intentional
poisoning in 47 cases, accidental poisoning in 20
cases, and an adverse reaction in one case. Overall, a
minor effect or no adverse effect occurred in 63 cases
(87.5%); a moderate effect occurred in five cases,
and a major effect occurred in four cases. Detailed
information regarding the four cases with major
effects is provided in Table 3. The age distribution
of affected individuals (among all 72 cases) is shown in Figure 4. Most poisoning cases (66.67%)
involving ADHD medications occurred in children
and adolescents; intentional poisoning occurred
in a much larger proportion of cases among older
individuals.
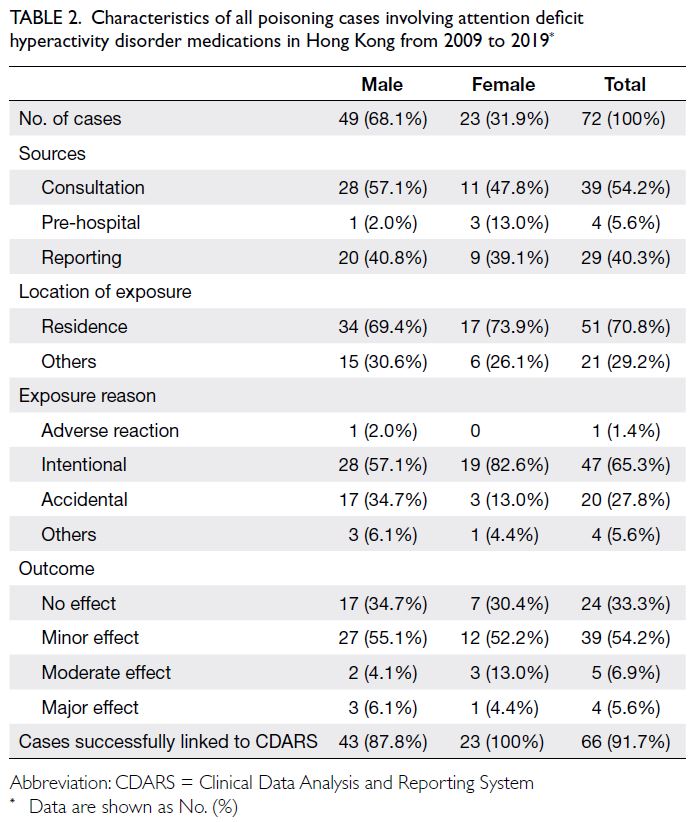
Table 2. Characteristics of all poisoning cases involving attention deficit hyperactivity disorder medications in Hong Kong from 2009 to 2019
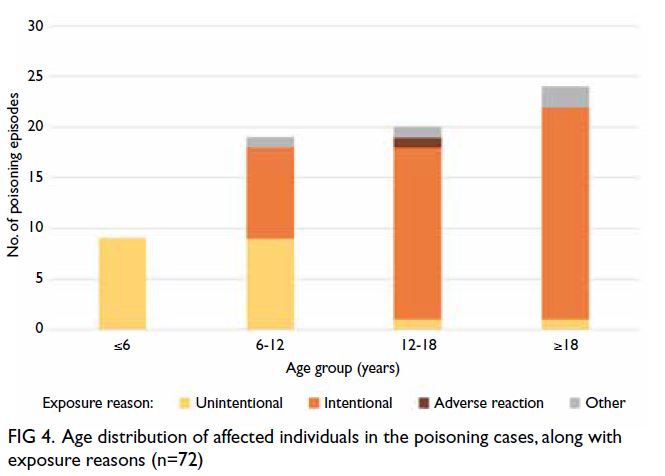
Figure 4. Age distribution of affected individuals in the poisoning cases, along with exposure reasons (n=72)
In total, 66 cases with an A<E number were
matched to CDARS data for 62 individuals (Table 4). Among the remaining six cases which were
not successfully linked, three did not have an
A<E number in the poisoning record. In total, 40
poisoning cases (median age: 14 years) occurred in 39
individuals with either an ADHD diagnosis or ADHD
prescription, and 26 poisoning cases (median age: 33
years) occurred in 23 individuals without ADHD.
The sources of poisoning reports and locations of
exposure were similar between the two groups. With
respect to exposure reason, there were more cases
of intentional poisoning among individuals without
ADHD (76.9% among individuals without ADHD vs
60.0% among individuals with ADHD); there were
also sex-related differences. Among female ADHD
patients and among both male and female non-ADHD individuals, more than three-quarters of
poisoning events were intentional. However, among
male ADHD patients, intentional and accidental
poisoning each constituted approximately half of all
cases. Furthermore, the distribution of poisoning
outcomes differed between individuals with and
without ADHD. Among individuals with ADHD, a
minor effect or no adverse effect occurred in most
cases; a major effect did not occur in any cases.
Among individuals without ADHD, a minor effect
occurred in >65% of cases; a major effect occurred
in four cases. The proportions of poisoning cases
caused by a single ADHD medication were 82.5%
among individuals with ADHD and 69.2% among
individuals without ADHD. Analysis of psychiatric
co-morbidities retrieved from the CDARS revealed
that individuals without ADHD more frequently
had mental disorders such as depression, anxiety, or
schizophrenia.
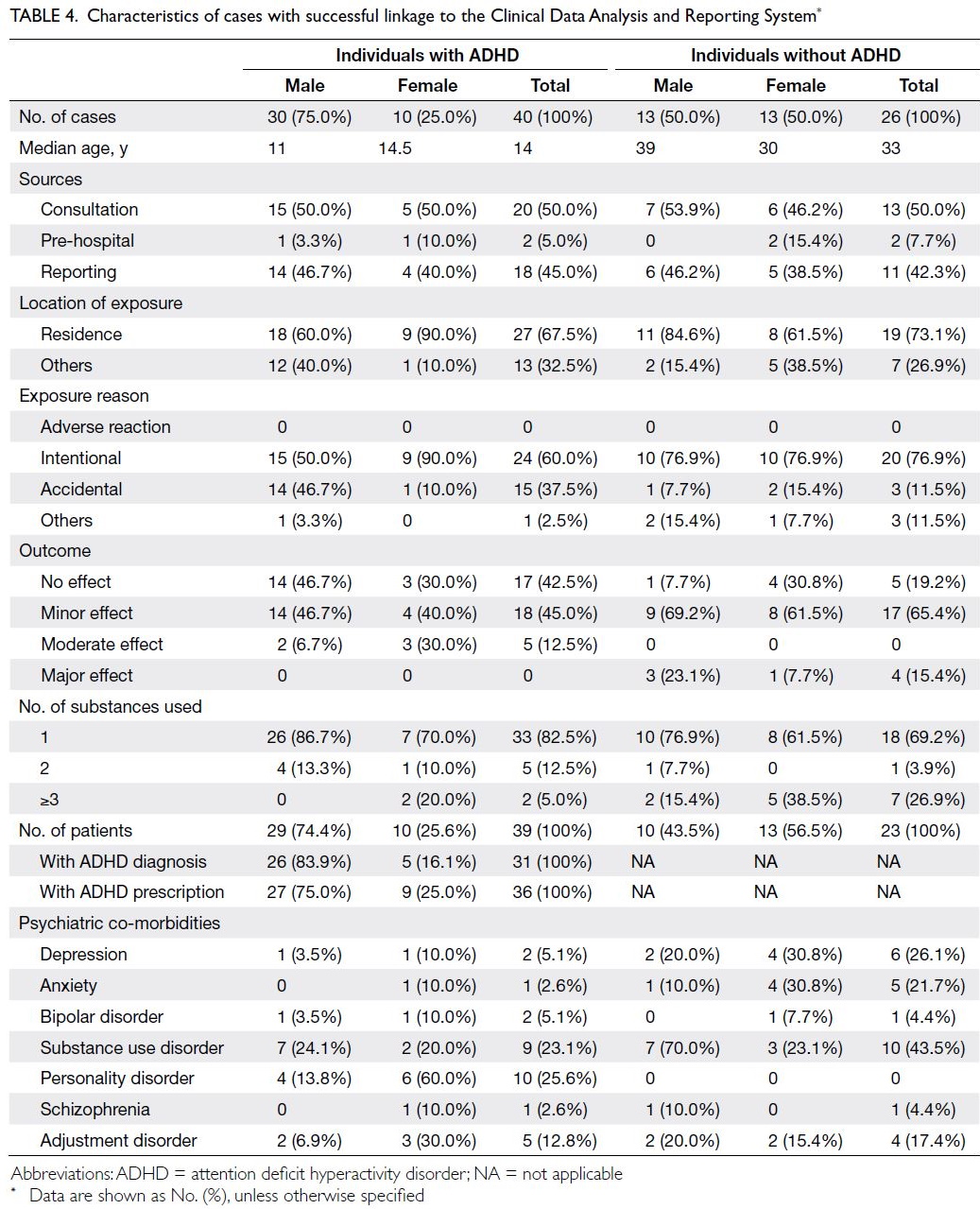
Table 4. Characteristics of cases with successful linkage to the Clinical Data Analysis and Reporting System
Discussion
Comparison with other studies
In this study, we analysed the characteristics of
poisoning cases involving ADHD medications
reported to the HKPIC between 2009 and 2019.
Overall, 72 cases were included in our analysis. The
most common location of poisoning was the affected
individual’s residence, and most cases occurred in
children and adolescents. These results are similar
to the findings in previous studies, including reports
from poison control centres of the US16 showing that
94.5% of cases occurred in the affected individual’s
residence, and a report from the Australian New
South Wales Poisons Information Centre17 revealing
a median age of 17 years among individuals with
intentional poisoning involving ADHD medications.
These results may be explained by the increased
likelihood of household exposure to various drugs, which increases the number of poisoning events
that occur in the affected individual’s residence.1
Additionally, children and adolescents are more
vulnerable to poisoning involving medications
because of their developmental progression, external
influences, and inadequate understanding of the
relevant dangers.27 According to the HKPIC 2018
annual report,11 more than half (64%) of poisoning events in that year were caused by exposure in the
affected individual’s residence, and 21.3% occurred
among individuals aged <20 years. Poisoning cases
occurred among individuals with ADHD at younger
ages, compared with cases among individuals without
ADHD; a potential explanation for this difference is
that, although ADHD is often a lifelong condition, it
is currently more commonly diagnosed in children and adolescents.28 Because of the limited number of
poisoning cases, we could not detect a statistically
significant correlation between trends in poisoning
cases and the number of ADHD prescriptions. The
authors of the New South Wales Poisons Information
Centre report17 compared intentional exposure to
ADHD medications with dispensing information for
those medications (using Pharmaceutical Benefits
Scheme data); their analysis revealed parallel trends.
Potential explanation of the findings
By using A<E numbers to link poisoning cases
with electronic health records from the CDARS,
we achieved a high linkage rate (91.7%). Among the
poisoning cases that occurred in male individuals
with ADHD, intentional and accidental poisoning
were equally common; however, among female
individuals with ADHD, the most common reason
for poisoning was self-harm. This distinction may
be related to sex differences in ADHD patients. For
example, girls and women are less likely than boys
and men to be diagnosed with ADHD; thus, female
ADHD patients may have severe symptoms or co-morbidities.29 30 However, with respect to poisoning
cases that occurred in individuals without ADHD,
exposure reasons were similar among male and female
individuals. Although >70% of the poisoning cases
were solely caused by ADHD medication, multiple
substances (eg, psychiatric medications or other
medications) had been used in some cases. Among
individuals without ADHD, there were more poisoning
cases that involved two or more types of substances.
This finding is presumably related to the higher risks
of mental disorders (eg, depression, anxiety, and
substance use disorders) among individuals without
ADHD; all of these mental disorders increase the risk
of intentional self-poisoning.31
Strengths and limitations
The main strength of this study is that, to our
knowledge, it is the first study in Asia to analyse
trends in ADHD prescriptions and poisoning
involving ADHD medications. Additionally, we
used A<E numbers to link data between the
HKPIC and the CDARS, yielding detailed co-morbidity
and prescription information for affected
individuals. The A<E number is a de-identified code
generated by the HA, which partially protects each
individual’s privacy. In this study, we made full use
of information available from various databases and
conducted preliminary analyses that will facilitate
future research.
However, there were some limitations in this
study. First, data from the HKPIC were collected
from the voluntary poisoning reporting system,11
which may not cover all poisoning cases involving
ADHD medications in Hong Kong. This type of
limitation is also present in other poison control or information centre reports,16 17 which may omit
cases of substance abuse, misuse, or overdose with
or without obvious clinical symptoms that are not
reported or not detected in electronic health records
databases. Additionally, the small sample size
limited the statistical power to identify correlations.
Second, although we linked HKPIC data to CDARS
data, we were only able to obtain all diagnoses and
medication records; we could not determine whether
subsequent interventions (ie, after poisoning events)
were implemented to prevent additional poisoning
cases. Finally, medications prescribed in private
clinics may not have been recorded, and we could
not obtain data regarding the use of unlicensed or
illegal medications. However, we expect the numbers
of such medications to be relatively low.22 23 Because
HA services are available to all Hong Kong residents32
and the majority of children and adolescents with
chronic conditions are under the care of the HA,33
our data are likely to be representative of ADHD
medications in Hong Kong; however, we currently
cannot determine the true rate of poisoning events
involving ADHD medications in Hong Kong.
Clinical implications
Generally, individuals with ADHD have a higher
risk of poisoning (both intentional and accidental).
Therefore, safe medication storage and management
strategies should be implemented to avoid poisoning
events involving ADHD medications.34 Regarding
individuals with ADHD, particularly children and
adolescents, proper caregiver training is necessary
to ensure the safe storage and reasonable disposal
of common household medicines.35 In the present
study, accidental poisoning events had occurred
in approximately 30% of poisoning cases involving
ADHD medications; most of these events occurred
among individuals aged <12 years. Thus, at least
one-quarter of poisoning cases could be prevented
by good medication storage strategies. Furthermore,
individuals with mental disorders should be
supported in the management of their prescriptions.
Appropriate psychological intervention and social/family support can also help to reduce the potential
for poisoning events.
Conclusion
No statistically significant correlation was evident between ADHD medication prescriptions and
poisoning events involving ADHD medications.
However, it remains important to raise awareness
regarding the management and safe storage of
medications among individuals with and without ADHD.
Author contributions
Concept or design: KKC Man, ML Tse, P Ip, ICK Wong.
Acquisition of data: L Gao, ATY Chow, CSL Chui, ML Tse, P Ip, ICK Wong.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: L Gao, KKC Man, KHTW Wong, EW Chan, CSL Chui.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: L Gao, ATY Chow, CSL Chui, ML Tse, P Ip, ICK Wong.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: L Gao, KKC Man, KHTW Wong, EW Chan, CSL Chui.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
L Gao, ATY Chow, ML Tse and KHTW Wong declare no
conflict of interest. KKC Man is a recipient of the CW
Maplethorpe Fellowship and reports grants from the National
Institute for Health Research of the United Kingdom,
Research Grants Council (RGC) of Hong Kong, Horizon
2020 Framework of the European Commission, and personal
fees from IQVIA Ltd, outside the submitted work. EW Chan
reports honorarium from the Hospital Authority, grants from
RGC, the Research Fund Secretariat of the Health Bureau
(formerly Food and Health Bureau) and the Narcotics Division
of the Security Bureau of the Hong Kong SAR Government,
the National Natural Science Fund of China, the Wellcome
Trust, Bayer, Bristol Myers Squibb, Pfizer, Janssen, Amgen,
and Takeda, outside the submitted work. CSL Chui reports
grants from Pfizer and personal fees from PrimeVigilance,
outside the submitted work. D Coghill reports grants and
personal fees from Shire/Takeda, and personal fees from
Medice, Servier and Oxford University Press, outside the
submitted work. As an editor of the journal, KL Hon was not
involved in the peer review process. P Ip reports research
grants from the RGC and the Health and Medical Research
Fund (HMRF) of the Hong Kong SAR Government, outside
the submitted work. ICK Wong reports research funding
outside the submitted work from Amgen, Bristol Myers
Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the RGC, the
HMRF, the National Institute for Health Research of the
United Kingdom, the European Commission and the National
Health and Medical Research Council in Australia, as well as
speaker fees from Janssen and Medice in the previous 3 years.
Funding/support
This work was supported by the Research Grants Council General Research Fund of the Hong Kong SAR Government
(Ref No.: 17125419) to KKC Man, ML Tse, EW Chan, CSL
Chui, D Coghill, KL Hon, P Ip and ICK Wong. The funder
had no role in study design, data collection/analysis/interpretation, or manuscript preparation.
Ethics approval
The study protocol was approved by the Research Ethics Committee of Kowloon Central Cluster/Kowloon East Cluster
(Ref No.: KC/KE-20-0173/ER-3) and the Institutional Review
Board of The University of Hong Kong/Hospital Authority
Hong Kong West Cluster (Ref Nos.: UW 20-779 and UW
12-136) of Hospital Authority, Hong Kong. The data presented
are fully anonymised, and the risk of identification is minimal.
This was a pharmacoepidemiology study without patient
contact and therefore informed consent was exempted by the
Research Ethics Committee/Institutional Review Board.
References
1. World Health Organization. World report on child injury prevention. 2008. Available from: https://www.who.int/publications/i/item/9789241563574. Accessed 23 May
2023.
2. Chan YC, Tse ML, Lau FL. Hong Kong Poison Information Centre: annual report 2009. Hong Kong J Emerg Med
2011;18:221-31. Crossref
3. Chan YC, Tse ML, Lau FL. Hong Kong Poison Information
Centre: annual report 2010. Hong Kong J Emerg Med
2012;19:110-20. Crossref
4. Chan YC, Tse ML, Lau FL. Hong Kong Poison Information
Centre: annual report 2011. Hong Kong J Emerg Med
2012;19:394-404. Crossref
5. Chan YC, Tse ML, Lau FL. Hong Kong Poison Information
Centre: annual report 2012. Hong Kong J Emerg Med
2013;20:371-81. Crossref
6. Chan YC, Tse ML, Lau FL. Hong Kong Poison Information
Centre: annual report 2013. Hong Kong J Emerg Med
2017;21:249-59. Crossref
7. Chan YC, Tse ML, Lau FL. Hong Kong Poison Information
Centre: annual report 2014. Hong Kong J Emerg Med
2017;22:376-87. Crossref
8. Chan YC, Tse ML, Lau FL. Hong Kong Poison Information
Centre: annual report 2015. Hong Kong J Emerg Med
2016;23:358-70. Crossref
9. Chan YC, Chan CK, Ng CH, Ng SH, Lau KK, Tse ML.
Hong Kong Poison Information Centre: annual report
2016. Hong Kong J Emerg Med 2017;24:244-54. Crossref
10. Lau KK, Chow AT, Chan CK, et al. Hong Kong Poison
Information Centre: annual report 2017. Hong Kong J
Emerg Med 2018;25:313-23. Crossref
11. Chow AT, Chan CK, Ng SH, Tse ML. Hong Kong Poison Information Centre: annual report 2018. Hong Kong J
Emerg Med 2020;27:344-55. Crossref
12. Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA.
The worldwide prevalence of ADHD: a systematic
review and metaregression analysis. Am J Psychiatry
2007;164:942-8. Crossref
13. Simon V, Czobor P, Bálint S, Mészáros A, Bitter I. Prevalence
and correlates of adult attention-deficit hyperactivity
disorder: meta-analysis. Br J Psychiatry 2009;194:204-11. Crossref
14. Ruiz-Goikoetxea M, Cortese S, Magallón S, et al. Risk
of poisoning in children and adolescents with ADHD: a
systematic review and meta-analysis. Sci Rep 2018;8:7584. Crossref
15. Raman SR, Man KK, Bahmanyar S, et al. Trends in
attention-deficit hyperactivity disorder medication use: a
retrospective observational study using population-based
databases. Lancet Psychiatry 2018;5:824-35. Crossref
16. King SA, Casavant MJ, Spiller HA, Hodges NL,
Chounthirath T, Smith GA. Pediatric ADHD medication
exposures reported to US poison control centers. Pediatrics
2018;141:e20173872. Crossref
17. Cairns R, Daniels B, Wood DA, Brett J. ADHD medication
overdose and misuse: the NSW Poisons Information
Centre experience, 2004-2014. Med J Aust 2016;204:154. Crossref
18. Lau WC, Cheung CL, Man KK, et al. Association between
treatment with apixaban, dabigatran, rivaroxaban, or
warfarin and risk for osteoporotic fractures among patients
with atrial fibrillation: a population-based cohort study.
Ann Intern Med 2020;173:1-9. Crossref
19. Wong AY, Wong IC, Chui CS, et al. Association between
acute neuropsychiatric events and Helicobacter pylori therapy containing clarithromycin. JAMA Intern Med 2016;176:828-34.Crossref
20. Lau WC, Chan EW, Cheung CL, et al. Association between dabigatran vs warfarin and risk of osteoporotic fractures among patients with nonvalvular atrial fibrillation. JAMA 2017;317:1151-8. Crossref
21. Man KK, Lau WC, Coghill D, et al. Association between
methylphenidate treatment and risk of seizure: a
population-based, self-controlled case-series study. Lancet
Child Adolesc Health 2020;4:435-43. Crossref
22. Man KK, Coghill D, Chan EW, et al. Association of risk of
suicide attempts with methylphenidate treatment. JAMA
Psychiatry 2017;74:1048-55. Crossref
23. Man KK, Chan EW, Coghill D, et al. Methylphenidate and
the risk of trauma. Pediatrics 2015;135:40-8. Crossref
24. Man KK, Coghill D, Chan EW, et al. Methylphenidate
and the risk of psychotic disorders and hallucinations in
children and adolescents in a large health system. Transl
Psychiatry 2016;6:e956. Crossref
25. Man KK, Chan EW, Ip P, et al. Prenatal antidepressant
use and risk of attention-deficit/hyperactivity disorder
in offspring: population based cohort study. BMJ
2017;357:j2350. Crossref
26. The Pennsylvania State University Eberly College of Science. STAT 510. Applied time series analysis. 8.2 Cross correlation functions and lagged regressions. Available
from: https://online.stat.psu.edu/stat510/lesson/8/8.2. Accessed 3 May 2021.
27. Parang M. What are common causes of poisoning in
children? Available from: https://www.webmd.com/children/what-are-common-causes-poisoning-in-children. Accessed 23 May 2023.
28. Scandurra V, Emberti Gialloreti L, Barbanera F, Scordo MR, Pierini A, Canitano R. Neurodevelopmental disorders and adaptive functions: a study of children with autism spectrum disorders (ASD) and/or attention deficit and hyperactivity disorder (ADHD). Front Psychiatry 2019;10:673. Crossref
29. Mowlem FD, Rosenqvist MA, Martin J, Lichtenstein P, Asherson P, Larsson H. Sex differences in predicting ADHD clinical diagnosis and pharmacological treatment. Eur Child Adolesc Psychiatry 2019;28:481-9. Crossref
30. Children and adults with attention-deficit/hyperactivity disorder (CHADD). Women and girls. Available from: https://chadd.org/for-adults/women-and-girls/. Accessed 28 Dec 2020.
31. Zhang J, Song J, Wang J. Adolescent self-harm and risk factors. Asia Pac Psychiatry 2016;8:287-95. Crossref
32. Chan EW, Lau WC, Leung WK, et al. Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: a population-based study. Gastroenterology 2015;149:586-95.e3. Crossref
33. Leung GM, Wong IO, Chan WS, Choi S, Lo SV; Health Care Financing Study Group. The ecology of health care in
Hong Kong. Soc Sci Med 2005;61:577-90. Crossref
34. Gao L, Man KK, Chan EW, et al. Treatment with
methylphenidate for attention deficit hyperactivity disorder
(ADHD) and the risk of all-cause poisoning in children and
adolescents: a self-controlled case series study. CNS Drugs
2021;35:769-79. Crossref
35. National Health Service, the United Kingdom Government. Poisoning - Prevention. Available from: https://www.nhs.uk/conditions/poisoning/prevention. Accessed 31 May 2023.





