Hong Kong Med J 2023 Apr;29(2):175–7 | Epub 17 Dec 2022
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
COMMENTARY
Detection of volatile organic compounds in exhaled breath for mass screening of COVID-19 infection
Christopher KC Lai, FHKCPath, FHKAM (Pathology)1; KW Choi, FHKAM (Medicine), FRCP (Lond)2; James Yao, P. Eng3; Raymond CF Tong, MD4
1 Department of Microbiology, The Chinese University of Hong Kong, Hong Kong
2 Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong
3 Massachusetts Institute of Technology, United States
4 Department of Medicine, The University of Toronto, Canada
Corresponding author: Dr Raymond CF Tong (rtlsjj@gmail.com)
Background
Large-scale mass screening of asymptomatic individuals is a commonly employed coronavirus disease 2019 (COVID-19) containment strategy. Regular screening is suggested for targeted groups such as airport and school staff,1 2 while population-scale mass screening is an integral component of a Zero-COVID-19 policy.3 Reverse transcriptase polymerase chain reaction (RT-PCR) and rapid antigen detection (RAT) tests have been widely deployed to detect SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) nucleic acid. Although RT-PCR has excellent sensitivity, it requires a long turnaround time of at least one hour for random access platforms and a few hours for batch tests. On the contrary, the RAT test has a quick turnaround time of 15 minutes, but sensitivity can be as low as 19% for individuals with a low viral low equivalent of cycle threshold (Ct) value >30.4 A novel screening tool that is sensitive, non-invasive, and has a rapid turnaround time with on-site results is needed.
Analysis of volatile organic compounds (VOC) in exhaled breath had been used previously to detect respiratory viruses such as human rhinovirus and influenza virus.5 6 Recent small-scale studies have already shown that detection of VOC in exhaled breath can differentiate COVID-19 from non-COVID-19 patients at first healthcare contact with >74% accuracy. 7 8 In another study, Grassin-Delyle et al9 identified VOC signatures that could differentiate between COVID-19 and non-COVID-19 acute respiratory distress syndrome with 93% accuracy. The United States Food and Drug Administration first authorised a COVID-19 diagnostic breath test in April 2022,10 and further consolidated the role of VOC detection in COVID-19 containment strategies. As such, a feasibility study to assess the use of such a test for COVID-19 screening in Hong Kong is urgently needed.
Methods
Subject recruitment
We performed a small-scale prospective pilot study and recruited subjects from the community. Healthy subjects with low pre-test probability were recruited through university student associations and workplaces where daily RAT was recommended by the prevailing government guidelines.1 They had no typical COVID-19 symptoms and had tested negative by RAT on the morning before the breath test. Subjects with high pre-test probability were recruited through social media and tested COVID-19 positive by RAT the same day as testing or were a contact of a confirmed case. Participation was voluntary with no financial incentive.
The RAT tests performed on the same day were either self-administered or performed at the testing facility. The RAT used was that available commercially or distributed by the government free of charge. All subjects had concomitant combined nasal and throat swabs collected by certified medical laboratory technicians that were submitted for RT-PCR tests for SARS-CoV-2 nucleic acid detection. The RT-PCR tests were performed by FlashDx-1000E (FlashDx Inc.) and served as the gold standard for determining COVID-19 infection status.
Sample collection using a novel volatile organic compound analyser
The VOC of exhaled breath from recruited subjects was collected by exhaling through a single-use disposable mouthpiece into the VOC analyser under direct supervision of certified medical laboratory technicians. The exhaled breath was sampled for 45 seconds using an array of built-in nanosensors. Subjects were notified of the result within minutes via an APP on a smartphone. The VOC analyser used was BreathPassTM (Deep Sensing Algorithms Ltd) which utilises a system of gas nanosensors to create a ‘snapshot’ of the breath print for detection and analysis of biomarkers found in volatile gases present in exhaled breath.
The device utilises probabilistic analysis and classification of subjects by detecting COVID-19-associated VOCs. The VOC pattern analysis is performed by an advanced Artificial Intelligence pattern recognition cloud algorithm. Each device is equipped with a high-intensity UVC system inside the sampling chamber as well as an electrostatic filter at the outlet to trap any virus that might escape.
Results
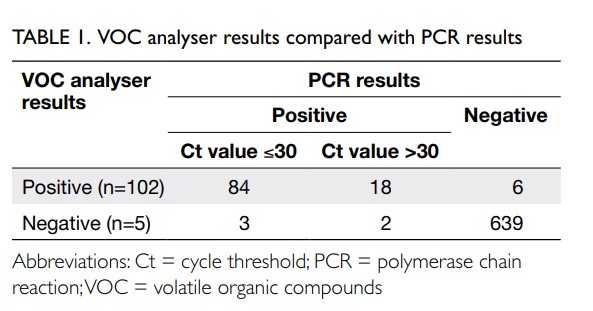
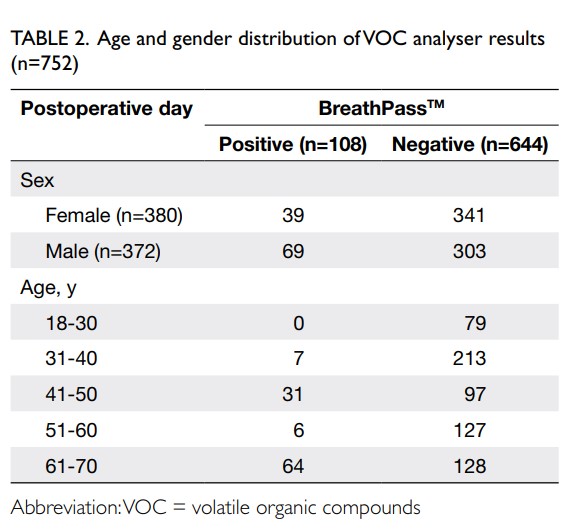
Between 26 September 2022 and 1 November 2022, 752 subjects aged 20 to 70 years were recruited of whom 372 (49.5%) were male. In all, 617 subjects with low pre-test probability were recruited from university student associations, laboratories, offices, and domestic helpers. There were no positive cases identified in this group and the results by VOC, RAT, and RT-PCR had 100% correlation. Among 107 antigen-positive COVID-19 subjects, the VOC analyser failed to detect five cases, the Ct values by PCR were all ≥29. For the 28 subjects who were close contacts, there were six false-positive cases by the VOC analyser that were subsequently confirmed negative by RT-PCR. On average, each operator could manage up to three devices simultaneously, offering a test rate of between 40 and 45 tests per hour, including the time required for performing hand hygiene, disinfection of surfaces, and changing disposable parts between subjects. The VOC analyser results were compared with the PCR results (Table 1). The age and gender distribution of VOC analyser results are summarised in Table 2. The overall sensitivity of the VOC analyser was 95.33% (95% confidence interval [95% CI=89.43-98.47%]), and specificity 99.07% (95% CI=97.99-99.66%). For low Ct value ≤30, the sensitivity and specificity were 96.55% (95% CI=90.25-99.28%) and 99.07% (95% CI=97.99-99.66%), respectively. For high Ct value >30, the sensitivity and specificity were 90.00% (95% CI=68.30-98.77%) and 99.07% (95% CI=97.99-99.66%), respectively. Assuming an overall prevalence of 1%, the negative predictive value was 99.95% (99.89-99.98%) and the positive predictive value was 50.87% (31.80-69.68%).
Discussion and conclusion
This is the first study to utilise a VOC analyser for COVID-19 testing in Hong Kong. Many VOC analysers are known to be affected by ambient conditions,11 including temperature and humidity, as well as chemicals such as alcohol, tobacco, hand sanitisers, and breath mints. Our device minimised the impact of these factors by a combination of proprietary sampling methodology and software normalisation. With the overall sensitivity and specificity >95%, our data are comparable to overseas data.7 8 11 Our study had several limitations. First, the sample size was small and importantly, we lacked data for those >70 years old. Second, we did not consider the possibility of co-infections, although the level of co-infection with other viruses in the community was extremely low during the study period. Third, our study included COVID-19 patients during the early phase of infection. The performance of our VOC analyser during a later stage of infection could not be ascertained. Finally, the study population was a convenient sample and may have been subject to selection bias. With our pilot findings, we recommend the use of VOC analysers for mass screening of COVID-19 in large populations with low-pretest probability.
Author contributions
All authors contributed to the concept or design of the study, acquisition, analysis, and interpretation of the data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
Authors have disclosed no conflicts of interest.
Funding/support
This study was privately funded by a donation from Dr Augustine Chow.
Ethics approval
This study was approved by The Chinese University of Hong Kong Survey and Behavioural Research Ethics Committee (Ref No. SBRE-22-0323). Informed consent was obtained from all subjects.
References
1. Hong Kong SAR Government. Community testing centres (CTCs) persons of targeted groups eligible for free COVID-19 nucleic acid testing service (with effect from 1 May 2022). Available from: https://gia.info.gov.hk/general/202204/30/P2022043000353_391761_1_1651311942469.pdf. Accessed 14 Nov 2022.
2. Infection Control Branch, Centre for Health Protection. Health advice on prevention of coronavirus disease (COVID-19) for school. Available from: https://www.chp.gov.hk/files/pdf/advice_to_school_on_prevention_of_nid_eng.pdf. Accessed 14 Nov 2022.
3. Wan Z, Lu R, Zhao Y, Zhang C. Diagnostic strategy of SARS-CoV-2 for containment under China’s zero-COVID-19 policy. J Infect 2022;85:e7-9. Crossref
4. Dinnes J, Sharma P, Berhane S, et al. Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev 2022;7:CD013705. Crossref
5. Schivo M, Aksenov AA, Linderholm AL, et al. Volatile emanations from in vitro airway cells infected with human rhinovirus. J Breath Res 2014;8:037110. Crossref
6. Aksenov AA, Sandrock CE, Zhao W, et al. Cellular scent of influenza virus infection. Chembiochem 2014;15:1040-8. Crossref
7. Ruszkiewicz DM, Sanders D, O’Brien R, et al. Diagnosis of COVID-19 by analysis of breath with gas chromatography-ion mobility spectrometry—a feasibility study. EClinicalMedicine 2020;29:100609. Crossref
8. Shan B, Broza YY, Li W, et al. Multiplexed nanomaterial-based sensor array for detection of COVID-19 in exhaled breath. ACS Nano 2020;14:12125-32. Crossref
9. Grassin-Delyle S, Roquencourt C, Moine P, et al. Metabolomics of exhaled breath in critically ill COVID-19 patients: a pilot study. EBioMedicine 2021;63:103154. Crossref
10. FDA News Release. Coronavirus (COVID-19) Update: FDA authorizes first COVID-19 diagnostic test using breath samples. 14 April 2022. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-covid-19-diagnostic-test-using-breath-samples. Accessed 14 Nov 2022.
11. Kwiatkowski A, Borys S, Sikorska K, Drozdowska K, Smulko JM. Clinical studies of detecting COVID-19 from exhaled breath with electronic nose. Sci Rep 2022;12:15990. Crossref