Hong Kong Med J 2023 Apr;29(2):173.e1–2
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PICTORIAL MEDICINE
A girl with acute-onset severe astigmatism and gaze palsy
KHA Kwok, MB, BS, MRCPCH1; KL Hon, MB, BS, MD1; CC Au, MB, BS, MRCPCH1; Wilson WS Ho, MB, BS, MRCS2; Elaine YL Kan, MB, ChB, FRCR3
1 Department of Paediatrics and Adolescent Medicine, Hong Kong Children’s Hospital, Hong Kong SAR, China
2 Department of Neurosurgery, Hong Kong Children’s Hospital, Hong Kong SAR, China
3 Department of Radiology, Hong Kong Children’s Hospital, Hong Kong SAR, China
Corresponding author: Dr KL Hon (ehon@hotmail.com)
 A video clip demonstrating physical examination findings of bilateral fixed left lateral gaze and right lower-motor-neuron seventh cranial nerve palsy is available at www.hkmj.org
A video clip demonstrating physical examination findings of bilateral fixed left lateral gaze and right lower-motor-neuron seventh cranial nerve palsy is available at www.hkmj.orgA previously healthy 4-year-old girl presented with
a 4-week history of acute-onset visual disturbance.
She was initially prescribed glasses for severe
astigmatism but vision did not improve. It was later
noted by her parents that her eyes could look only
to the left side. She was then admitted to hospital.
There was no history of headache, photophobia or vomiting but she exhibited drooling from the
right side of her mouth and unsteady gait. She
was afebrile and all other vital signs were normal
with Glasgow Coma Scale score of 15. Physical
examination revealed bilateral fixed left lateral gaze
and right lower-motor-neuron seventh cranial nerve
palsy. When asked to look to her right, she needed
to compensate by head turning. Pupils were 3 mm
equal and reactive to light. Ear canals were normal
and there was no skin rash. Computed tomography
scan of the brain showed a multilobulated mixed
hyperdense and hypodense lesion at the dorsal pons
with compression of the fourth ventricle. Magnetic
resonance imaging (MRI) of the brain revealed
bleeding from a tumour at the right dorsal pons
(Fig 1); overall picture suggested multiple
cavernoma (Fig 2). Neurosurgical decompression
and stereotactic excision of a brainstem lesion was
performed via suboccipital craniotomy. Histology
showed features of a vascular lesion in keeping with
cavernous haemangioma. Intracranial pressure was
monitored via an external ventricular drain and
remained normal postoperatively. Postoperative
neurological examination showed bilateral fixed
left lateral gaze, right lower-motor-neuron seventh cranial nerve palsy, and contralateral weakness of
limbs. Genetic testing demonstrated a pathogenic
mutation [heterozygous KRIT1 c.690C>A
p.(Tyr230*)] for familial cerebral cavernous
malformation (CM) syndrome. Her family was
referred for further genetic testing and counselling.
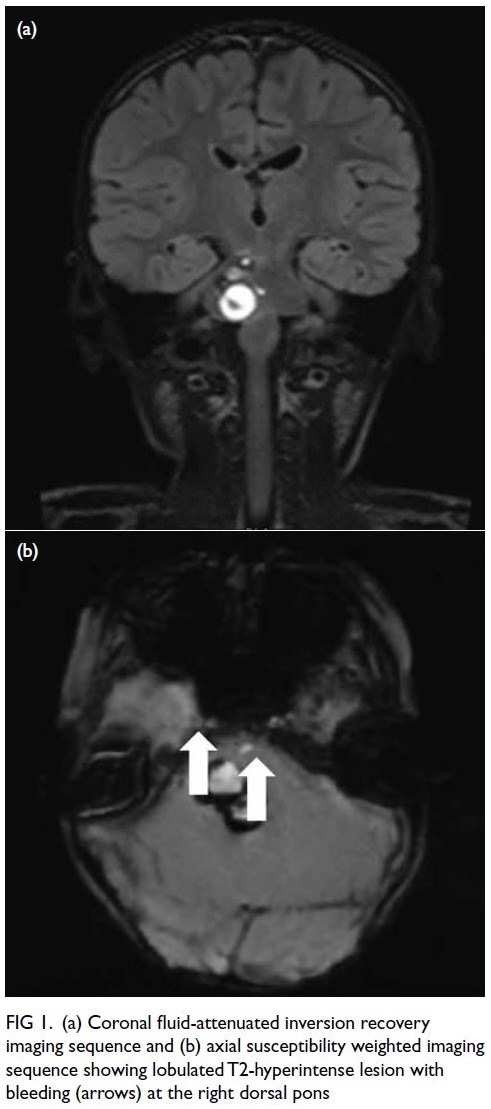
Figure 1. (a) Coronal fluid-attenuated inversion recovery imaging sequence and (b) axial susceptibility weighted imaging sequence showing lobulated T2-hyperintense lesion with bleeding (arrows) at the right dorsal pons
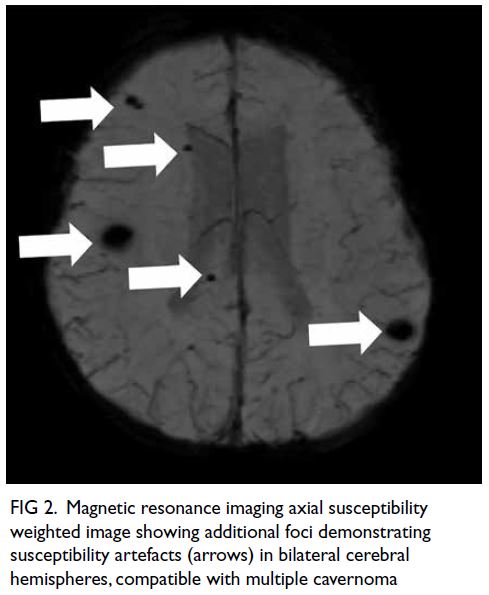
Figure 2. Magnetic resonance imaging axial susceptibility weighted image showing additional foci demonstrating susceptibility artefacts (arrows) in bilateral cerebral hemispheres, compatible with multiple cavernoma
Acute-onset gaze disturbance in children
is alarming and may signify serious cerebral
abnormality. Almost all conjugate gaze palsies
originate from a lesion in the midbrain or pons. These
lesions can be caused by vascular or oncological
space occupying lesions.
Cavernous malformations, also known as
cavernous haemangiomas, are a type of benign,
congenital malformation in which a cluster of
dilated thin-walled capillaries form a characteristic
‘mulberry’ lesion with engorged purplish colour.1
They can be sporadic or inherited with an autosomal
dominant pattern and incomplete penetrance,
and can present as solitary or multiple lesions.
Unlike capillary haemangiomas, CMs can be life-threatening
and do not tend to regress. In all, 25%
of cerebral CMs are infratentorial. They have a
bleeding rate of 2% to 3% per year and recurrent
bleeding rate of >20%. Due to the close proximity
to multiple brainstem nuclei and fibre tracts,
progressive neurological decline is observed in
39% patients with infratentorial CMs.2 Magnetic
resonance imaging is the modality of choice,
and susceptibility weighted imaging is the most
sensitive means to detect blood products thus key to
diagnosing cerebral CMs. Evolving blood products
inside a CM appear as variable image intensities and
give rise to the typical ‘popcorn’ appearance.3 Since
MRI appearance is usually pathognomonic, biopsy is
rarely needed. Angiography is indicated only if MRI
cannot exclude arteriovenous malformation. Genetic
testing should be arranged to screen for familial
cerebral CM. Treatment approach depends on the
site, size, symptoms, and history of haemorrhage.4
Indications for surgical resection of a cerebral CM
include intracranial haemorrhage and epilepsy.
Options include conventional surgery or stereotactic
radiosurgery.4 5
Author contributions
All authors contributed to the concept or design, acquisition of data, analysis or interpretation of data, drafting of the
manuscript, and critical revision of the manuscript for important intellectual content.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
As an editor of the journal, KL Hon was not involved in the peer review process. Other authors have disclosed no conflicts
of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Ethics approval for this study was obtained from Hong Kong Children’s Hospital Ethics Committee, Hong Kong (Ref No.:
HKCH REC 2019 009). The patient was treated in accordance
with the tenets of the Declaration of Helsinki. Written consent
for publication has been obtained from the patient’s parent.
References
1. Awad IA, Polster SP. Cavernous angiomas: deconstructing a neurosurgical disease. J Neurosurg 2019;131:1-13. Crossref
2. Fritschi JA, Reulen HJ, Spetzler RF, Zabramski JM. Cavernous malformations of the brain stem. a review of
139 cases. Acta Neurochir (Wien) 1994;130:35-46. Crossref
3. Lehnhardt FG, von Smekal U, Rückriem B, et al. Value of gradient-echo magnetic resonance-imaging in the
diagnosis of familial cerebral cavernous malformation.
Arch Neurol 2005;62:653-8. Crossref
4. Poorthuis MH, Klijn CJ, Algra A, Rinkel GJ, Al-Shahi Salman R. Treatment of cerebral cavernous malformations:
a systematic review and meta-regression analysis. J Neurol
Neurosurg Psychiatry 2014;85:1319-23. Crossref
5. Wang CC, Liu A, Zhang JT, Sun B, Zhao YL. Surgical management of brain-stem cavernous malformations:
report of 137 cases. Surg Neurol 2003;59:444-54. Crossref

