Hong Kong Med J 2023 Apr;29(2):105–11 | Epub 30 Mar 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Efficacy, toxicities, and prognostic factors of
stereotactic body radiotherapy for unresectable liver metastases
Calvin KK Choi, FHKCR, FHKAM (Radiology); Connie HM Ho, FHKCR, FHKAM (Radiology); Matthew YP Wong, MSc; Ronnie WK Leung, MSc; Frank CS Wong, FHKCR, FHKAM (Radiology); Stewart Y Tung, FHKCR, FHKAM (Radiology); Francis AS Lee, FHKCR, FHKAM (Radiology)
Department of Clinical Oncology, Tuen Mun Hospital, Hong Kong SAR, China
Corresponding author: Dr Calvin KK Choi (calvinkkchoi@hkbh.org.hk)
Abstract
Introduction: This study aims to determine the outcomes of stereotactic body radiotherapy (SBRT) for liver metastases in patients not eligible for surgery.
Methods: This study included 31 consecutive
patients with unresectable liver metastases who
received SBRT between January 2012 and December
2017; 22 patients had primary colorectal cancer and
nine patients had primary non-colorectal cancer.
Treatments ranged from 24 Gy to 48 Gy in 3 to 6
fractions over 1 to 2 weeks. Survival, response rates,
toxicities, clinical characteristics, and dosimetric
parameters were evaluated. Multivariate analysis
was performed to identify significant prognostic
factors for survival.
Results: Among these 31 patients, 65% had
received at least one prior regimen of systemic
therapy for metastatic disease, whereas 29% had
received chemotherapy for disease progression or
immediately after SBRT. The median follow-up
interval was 18.9 months; actuarial in-field local
control rates at 1, 2, and 3 years after SBRT were
94%, 55%, and 42%, respectively. The median survival
duration was 32.9 months; 1-year, 2-year, and 3-year
actuarial survival rates were 89.6%, 57.1%, and
46.2%, respectively. The median time to progression was 10.9 months. Stereotactic body radiotherapy
was well-tolerated, with grade 1 toxicities of fatigue
(19%) and nausea (10%). Patients who received post-SBRT chemotherapy had significant longer overall
survival (P=0.039 for all patients and P=0.001 for
patients with primary colorectal cancer).
Conclusion: Stereotactic body radiotherapy can be
safely administered to patients with unresectable
liver metastases, and it may delay the need for
chemotherapy. This treatment should be considered
for selected patients with unresectable liver
metastases.
New knowledge added by this study
- Stereotactic body radiotherapy (SBRT) for unresectable liver metastases was effective and well-tolerated. It may delay the need for chemotherapy while prolonging progression-free survival.
- The receipt of post-SBRT chemotherapy is a significant prognostic factor for survival.
- Stereotactic body radiotherapy can be regarded as an alternative to surgery for patients with liver metastases, particularly patients with unresectable tumours.
- We recommend offering SBRT to patients with unresectable liver metastases if they have good performance status (ie, Eastern Cooperative Oncology Group 0-1), liver tumours ≤6 cm in diameter, three or fewer liver tumours, normal liver volume >700 cm3, adequate organ function, and adequate liver function (Child-Pugh class A).
Introduction
The liver is a common site of metastases, which
most frequently originate from primary colorectal
cancer via portal circulation. Surgical resection is
the standard treatment for medically and technically
operable liver metastases, particularly from primary colorectal cancer. However, most patients are
not eligible for surgery because of co-morbidities
or unfavourable tumour factors. Most patients
receive systemic therapy as initial treatment for
liver metastases, but such treatment rarely leads
to permanent elimination of the metastases; some form of local ablative intervention is required. For
patients with unresectable limited liver metastases,
numerous local therapeutic approaches are available,
such as radiofrequency ablation, transcatheter
arterial chemoembolisation, cryotherapy, and high-intensity
focal ultrasound. However, all of these
approaches exhibit a degree of invasiveness and are
currently limited by tumour size (usually <3 cm),
distance from critical structures, and distance from
critical vasculature.1
In the past, radiotherapy has had a limited role
in the management of liver metastases because of
concerns regarding radiation-induced liver disease.2 3
Because the liver is subject to the parallel architecture
principles of radiobiology, the risk of radiation-induced
liver disease is generally proportional to
the mean dose of radiation delivered to normal liver
tissue. Therefore, small hepatic lesions can be safely
treated with high doses of radiation via stereotactic
body radiotherapy (SBRT). Advances in tumour
imaging, radiotherapy planning and delivery, and
motion management have facilitated the delivery
of highly precise and four-dimensional SBRT.
This non-invasive method can be used to deliver
ablative treatments on an outpatient basis, thereby
decreasing morbidity and cost.4
Ablative techniques offer a minimally
invasive treatment option for selected patients with oligometastatic liver disease.5 There is increasing
evidence to support the use of SBRT.6 To our
knowledge, there is limited published information
regarding the role of SBRT in the treatment of
unresectable liver metastases in Hong Kong. In
this study, we investigated the efficacy, toxicities,
and prognostic factors of SBRT in patients with
unresectable liver metastases.
Methods
Patient eligibility
Data regarding consecutive patients with
unresectable liver metastases who received SBRT
between January 2012 and December 2017 were
retrospectively retrieved from the treatment
database of the Department of Clinical Oncology
at Tuen Mun Hospital. All patients with liver
metastases were evaluated in multidisciplinary
team meetings involving radiation oncologists and
hepatobiliary surgeons. Eligibility was determined
using the following criteria: (1) histologically
confirmed malignancy (hepatic lesion biopsy not
required); (2) biphasic computed tomography
(CT) scan or positron emission tomography–CT
of the liver within 4 weeks of radiation planning
demonstrating liver tumours ≤6 cm in diameter,
presence of three or fewer liver tumours, and normal
liver volume >700 cm3; (3) discussion of the case
in a multidisciplinary team meeting that included
an opinion regarding the lack of qualification for
radiofrequency ablation, along with a determination
of non-resectability by a qualified hepatic surgeon;
(4) patient refusal of surgical treatment; (5) Eastern
Cooperative Oncology Group performance status 0
or 1; (6) adequate organ function (absolute neutrophil
count ≥1.5×109/L; platelet count ≥75×109/L;
creatinine level ≤1.5×upper limit of normal), liver
function test results (aspartate aminotransferase
and alanine aminotransferase levels ≤1.5×normal
level), and Child-Pugh score of ≤6 (class A); (7)
controlled extrahepatic disease and life expectancy
>6 months; (8) no chemotherapy concurrent with
radiotherapy (previous chemotherapy was not an
exclusion criterion); and (9) previous treatment
with radiofrequency ablation was not an exclusion
criterion if recurrence had been confirmed.
Radiotherapy treatment
During four-dimensional CT scans, patients were
positioned supine on an evacuated foam bag (Klarity
Medical, China) with both arms abducted. The extent
of tumour motion during respiration was used to
determine whether treatment would be administered
with free breathing plus abdominal compression or
active breathing control. The gross tumour volume
(GTV) was determined using contrast CT and co-registered
with positron emission tomography–CT. For patients who required optimal abdominal
compression to mitigate organ motion, planning
was conducted using the mid-ventilation–based
planning target volume (PTV) approach, and the
GTV was determined using intravenous contrast
CT. The clinical target volume was 0 mm outside
of the GTV within the liver (ie, equal to GTV); it
included the position of the tumour in all phases
of respiration. The PTV was defined by adding an
isotropic margin of 3 to 5 mm from the clinical target
volume or 7 to 10 mm in the cranial-caudal axis and
4 to 6 mm in the anterior-posterior and lateral axes.
Pretreatment four-dimensional cone-beam CT was
performed prior to each treatment for all patients to
adjust for setup uncertainties. Tumour localisation
was conducted using the diaphragm or whole liver
as a surrogate for the tumour. A two-step four-dimensional
registration approach was used to align
the diaphragm/liver surrogate to its time-weighted
mean position. The SBRT dose, ranging from 8 to
16 Gy × 3 fractions to 5 to 7.5 Gy × 6 fractions, was
individualised according to the following normal
tissue constraints: (1) maximum spinal cord dose
<15 Gy; (2) ≥700 cm3 of liver should receive <15 Gy,
and D5% <30 Gy; (3) maximum stomach point dose
of 25 Gy; and (4) maximum duodenum point dose
of 25 Gy.
Evaluation
Patients were evaluated weekly during SBRT,
immediately after completion of treatment, at 6
weeks after treatment, every 3 months for the first
2 years, and every 4 months thereafter. Physical
examinations and blood tests were performed at
each follow-up visit. Triphasic CT of the liver was
conducted at 3 months after SBRT and then every 6
months until disease progression. Tumour response
was assessed using modified response evaluation
criteria for solid tumours.
The primary endpoint of the study was local
control; secondary endpoints were overall survival
and toxicity. Local control was defined as the
absence of progressive disease within the PTV. The
appearance of new lesions outside of the PTV was
regarded as intrahepatic out-field failure. Overall
survival was calculated from the start of SBRT until
the end of follow-up or death.
Toxicity was graded using the National Cancer
Institute Common Terminology Criteria for Adverse
Events version 4.0. Toxicities were defined as adverse
events that occurred <3 months after SBRT. Newly
developed toxicities or toxicities that progressed to
one grade above baseline were regarded as adverse
events. Grade 5 liver failure related to SBRT was
defined as death from liver failure in the presence
of acute grade 3 liver toxicities during <6 months
without intrahepatic progression.
Statistical analysis
Data were analysed using SPSS software (Windows
version 23.0; IBM Corp, Armonk [NY], United
States). Fisher’s exact test and independent t tests
were used for univariate analysis of patient, disease,
and treatment factors associated with liver toxicity.
Binary logistic regression analysis was used for univariate analysis of dose-volumetric parameters
associated with liver toxicity. Kaplan–Meier test
was used for univariate analysis of overall survival,
with a significance threshold of P<0.25; it was used
for multivariate analysis of overall survival, with a
significance threshold of P<0.05. Cox regression was
used for further evaluation of variables which were
significant in univariate analysis of overall survival.7 8
Results
Patients and treatment
During the study period, 31 consecutive patients
with unresectable liver metastases underwent SBRT
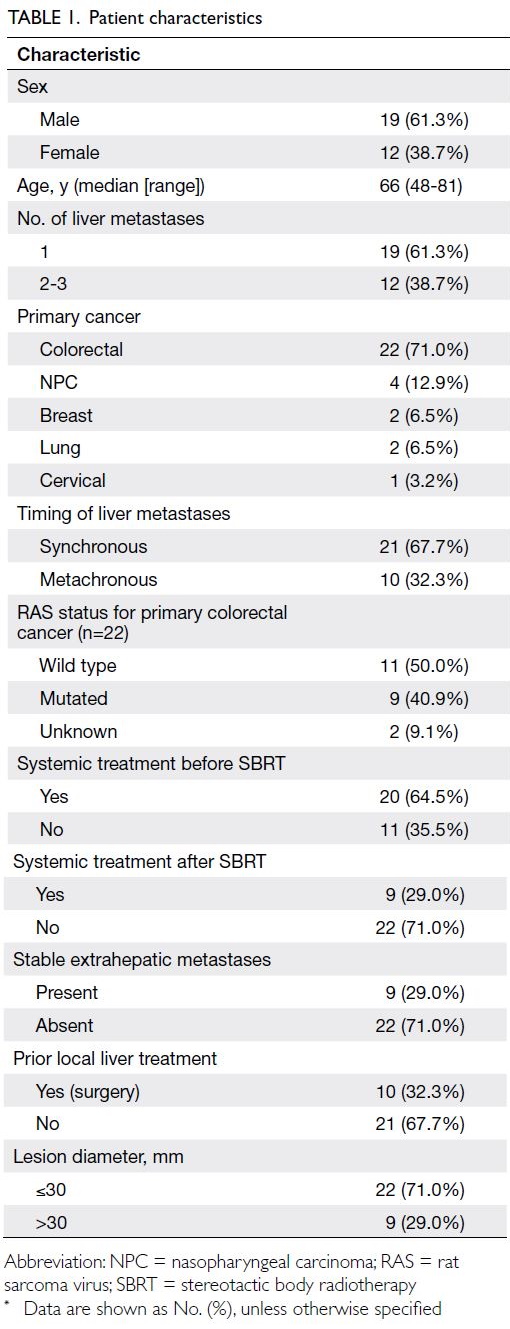
at our institution. Their characteristics are shown in
Table 1. Colorectal cancer was the most common
primary cancer. A total of 64.5% of patients received
systemic treatment before SBRT; 71% of liver lesions
were ≤ 30 mm. All patients received a fixed course
of 3 or 6 fractions with total prescribed dose ranges
of 24-48 Gy. The mean GTV was 26.9 cm3 (range,
1.5-137) and mean PTV was 91.8 cm3 (range, 21.7-269). The mean biological equivalent dose (BED10)
to GTV was 79.8 Gy (range, 43.2-124.8). The median
BED10 to GTV was 76.8 Gy. Surgical resection or
radiofrequency ablation were performed in 32%
of patients before SBRT. Targeted or non-targeted
systemic chemotherapy was administered to 65% and
29% of patients before and after SBRT, respectively.
Toxicities
Stereotactic body radiotherapy was well-tolerated.
There were no grade 2-4 toxicities. Most patients
were asymptomatic (grade 0) during radiotherapy;
19% of patients had grade 1 fatigue, 10% of patients
had grade 1 nausea, and 3% of patients had skin
reaction. No patients exhibited a change in Child-Pugh class after SBRT, and no significant prognostic
factors for liver toxicities were identified.
Local control, survival, and prognostic
factors
The median follow-up interval was 18.9 months. The
1-year, 2-year, and 3-year local control rates were
94% (29/31), 55% (17/31) and 42% (13/31),
respectively. Only two patients (9% of all patients)
with primary colorectal cancer had in-field
recurrence at 1 year after SBRT. Sixteen patients
in all treatment groups had out-field recurrence at
1 year after SBRT. The median time to progression
was 10.9 months.
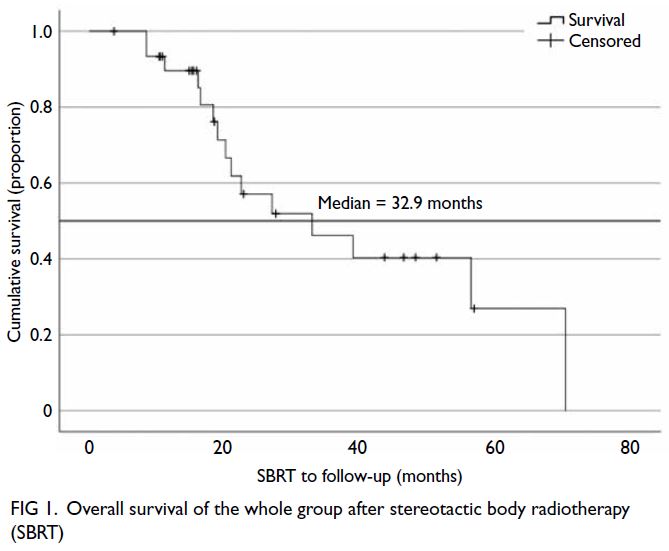
The median survival duration in all treatment
groups was 32.9 months. The 1-year, 2-year, and
3-year survival rates were 89.6%, 57.1%, and 46.2%,
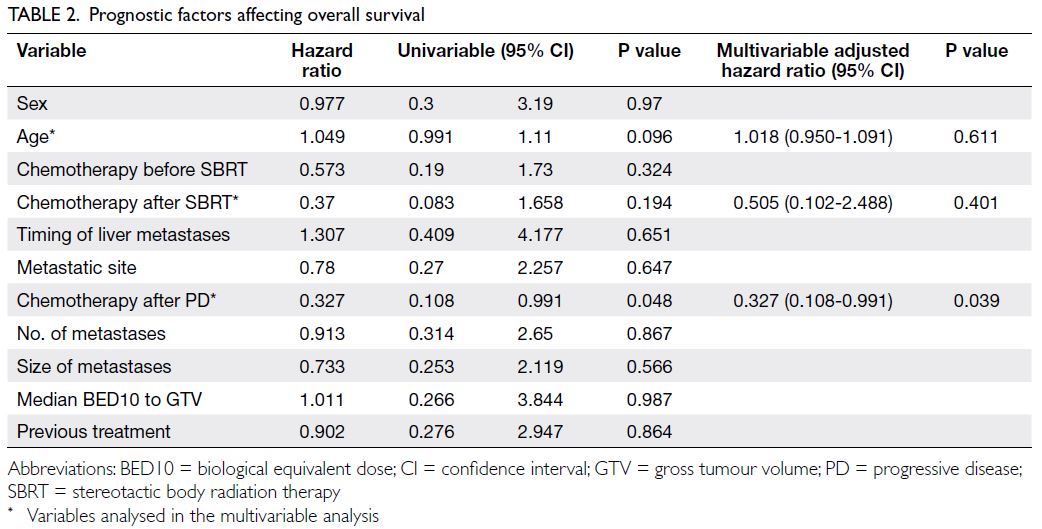
respectively. The only significant prognostic factor
for overall survival was receipt of post-SBRT chemotherapy for disease progression (P=0.039). Figures 1 and 2 show the survival curves and prognostic factors
for all treatment groups. Previous local treatment,
rat sarcoma virus status of colorectal cancer, number
of liver metastases, extrahepatic metastases, BED
to the liver, extrahepatic metastasis status, number
of chemotherapy lines before or after SBRT, and
carcinoembryonic antigen level after SBRT were not
significant prognostic factors for overall survival.
Table 2 summarises the factors that affected overall survival.
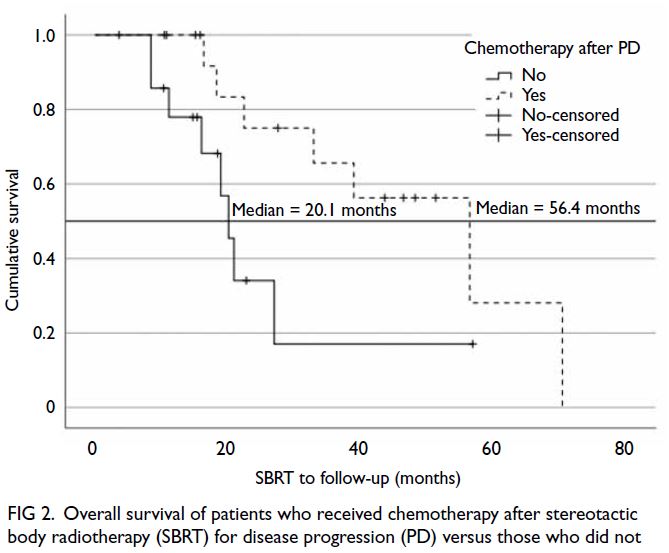
Figure 2. Overall survival of patients who received chemotherapy after stereotactic body radiotherapy (SBRT) for disease progression (PD) versus those who did not
The median survival duration in the colorectal
cancer subgroup was 32.9 months. The only
significant prognostic factor for overall survival
was receipt of post-SBRT chemotherapy for disease progression (P=0.001). No other significant
prognostic factors for overall survival were
identified. Figures 3 and 4 show the survival curves
and prognostic factors for the colorectal cancer subgroup.
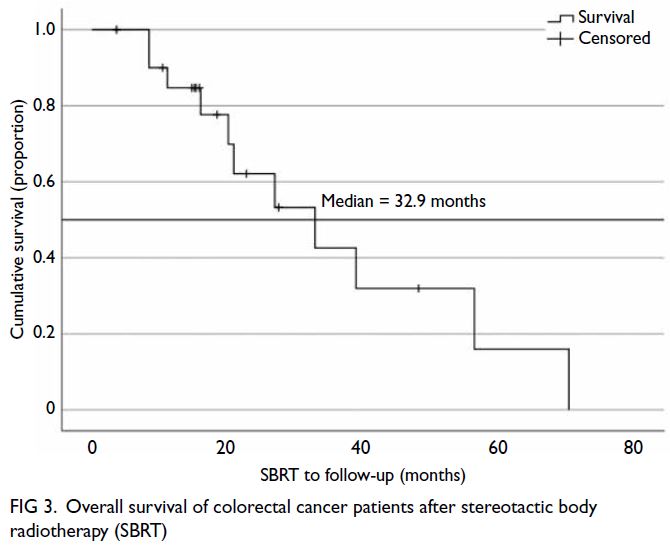
Figure 3. Overall survival of colorectal cancer patients after stereotactic body radiotherapy (SBRT)
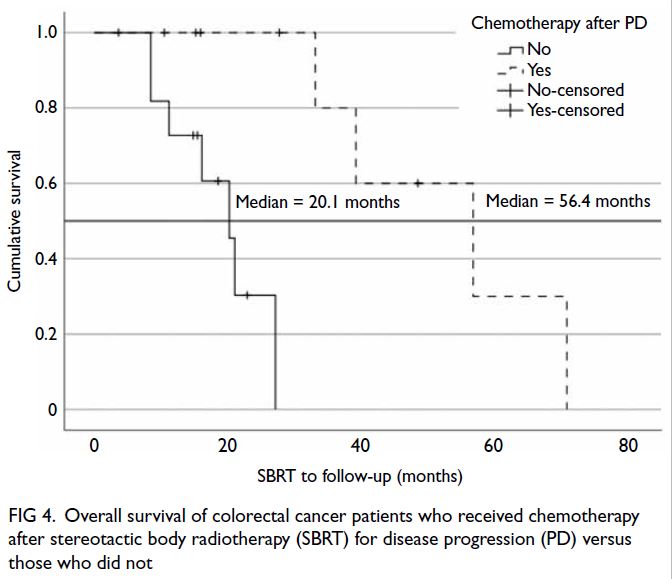
Figure 4. Overall survival of colorectal cancer patients who received chemotherapy after stereotactic body radiotherapy (SBRT) for disease progression (PD) versus those who did not
Discussion
Although surgical resection is the standard
treatment for liver metastases, many patients are not
eligible for such treatment. Multiple retrospective
and prospective studies have demonstrated SBRT
is a promising, safe, and non-invasive alternative to
surgery for unresectable liver metastases.9 10 To our
knowledge, there is limited published information
regarding the use of SBRT to treat liver metastases in
Hong Kong. In the present study, we retrospectively
collected data regarding consecutive patients who
received SBRT for unresectable liver metastases
after multidisciplinary team evaluation; we assessed
outcomes in terms of safety, local control, and
survival. Among the 31 patients treated with SBRT,
the 1-year and 2-year local control rates were 93%
and 55%, respectively. The median survival duration
was 32.9 months; the 1-year and 2-year survival rates
were 89.6% and 57.1%, respectively. In the colorectal
cancer subgroup, the 1-year and 2-year survival rates
were 84.7% and 62.1%, respectively.
Multiple retrospective and prospective studies
have been performed regarding SBRT for liver
metastases from colorectal cancers (Table 3).11 12 13 14 In the present study, local control rates and survival rates were comparable with findings in previous reports. Notably, McPartlin et al11 conducted a prospective study using SBRT doses of 22-62 Gy
in 6 fractions. The present study, with SBRT doses
of 24-48 Gy in 3-6 fractions, demonstrated better
1-year local control (93% vs 50%) and 2-year survival
(62.1% vs 26%) than the study by McPartlin et al.11
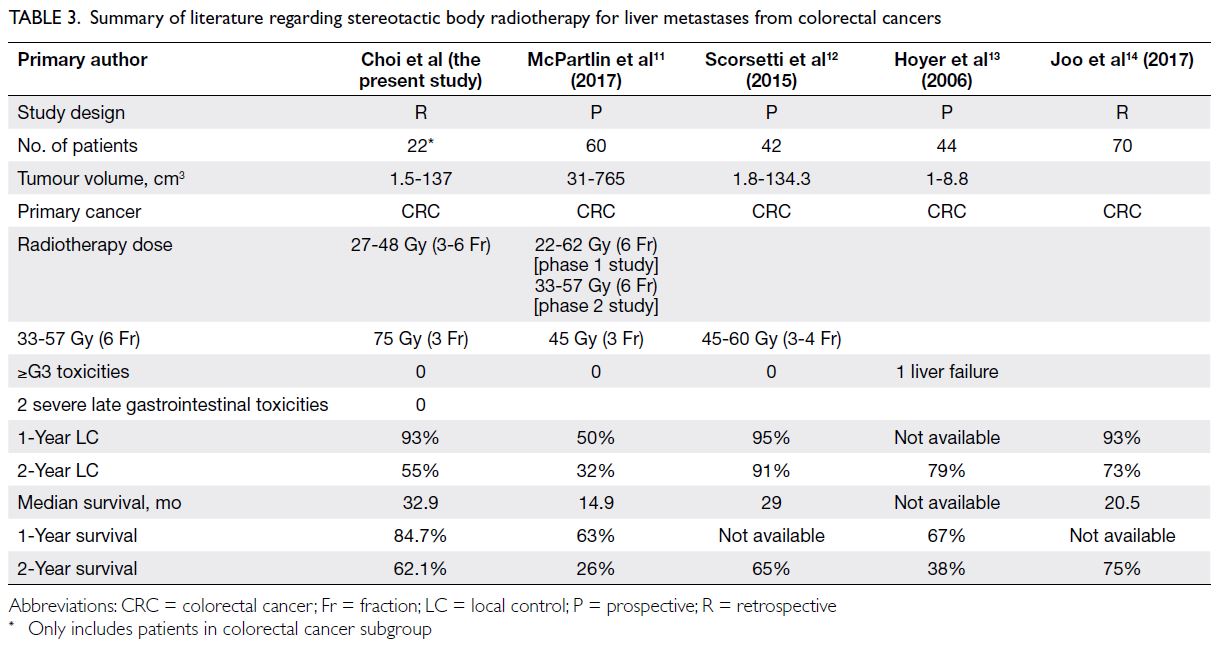
Table 3. Summary of literature regarding stereotactic body radiotherapy for liver metastases from colorectal cancers
Three other SBRT trials12 13 14 (45-75 Gy in 3
fractions) all demonstrated better local control rates
than the findings in the present study (Table 3).
These results indicate that a higher local control rate is associated with a higher radiation dose. Compared
with the present study, Scorsetti et al12 and Joo et al14
showed higher 2-year survival rates (65% and 75%,
respectively vs 62.1% in the present study), whereas
Hoyer et al13 revealed a considerably lower 2-year survival rate (38%). These discrepant findings may be related to radiation dose—Scorsetti et al12 and Joo et al14 reported higher BED than that achieved by
Hoyer et al13 and the present study. Among patients
with primary colorectal tumours, the survival rate
in the present study was comparable with rates in
the previous studies.11 12 13 14 However, overall survival
is dependent on many factors other than local
control of irradiated liver metastases. Compared
with earlier studies, overall survival is expected to
be better in more recent studies because of stage
migration, improvements in diagnostic techniques,
and enhanced systemic treatment. Importantly,
although the present study showed that post-SBRT
chemotherapy was a prognostic factor for longer
survival, selection bias may have been involved in
the decision to administer chemotherapy to patients
with better performance status.
In the present study, the incidence of toxicities
was low, and there were no grade 2-4 toxicities.
Among patients who received SBRT, only grade 1
toxicities were reported (fatigue, nausea, and skin
reaction); these findings indicate that SBRT was
well-tolerated.
Based on our results, we recommend that
patients with unresectable liver metastases are
evaluated in multidisciplinary team meetings;
patients should be offered SBRT if they have
good performance status (ie, Eastern Cooperative
Oncology Group 0-1), liver tumours ≤6 cm in
diameter, three or fewer liver tumours, normal
liver volume >700 cm3, adequate organ function, and adequate liver function (Child-Pugh class A).
Considering its minimal invasiveness and toxicity, as
well as its potential for improving progression-free
survival, SBRT should be regarded as an alternative
to surgical resection of liver metastases to those
patients who refuse surgical treatment.
There were some limitations in the present
study. First, the BED to the tumour was low (median
BED10 >100 Gy was administered to 35.5% of
patients), and the mean GTV was high (26.9 cm3).
The local control rate may have been influenced
by the lower total radiation dose administered
and larger tumour volume. Second, this was a
retrospective study, and the sample size was small.
Thus, a randomised controlled trial with a large
number of patients is needed to determine whether
SBRT can prolong overall survival in patients with
liver metastases.
Conclusion
Stereotactic body radiotherapy can be safely
administered to patients with unresectable
liver metastases, and it may delay the need for
chemotherapy. Considering its minimal invasiveness
and toxicity, this treatment should be offered to
selected patients with unresectable liver metastases;
such an approach may improve progression-free
survival. A phase III randomised study is needed to
confirm these results.
Author contributions
All authors contributed to the concept or design of the study, acquisition of data, analysis or interpretation of data, drafting of the manuscript, and critical revision of the manuscript for
important intellectual content. All authors had full access to
the data, contributed to the study, approved the final version
for publication, and take responsibility for its accuracy and
integrity.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgement
The authors thank Mr Jia-jie Huang from Quality and Safety Division of New Territories West Cluster, Hospital Authority,
Hong Kong for his statistical analysis support.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Ethics approval document was issued by New Territories West Cluster Research Ethics Committee of Hospital Authority,
Hong Kong (Ref No.: NTWC/REC/20035). Informed consent
was obtained from patients for stereotactic body radiotherapy.
References
1. Aitken KL, Hawkins MA. Stereotactic body radiotherapy for liver metastases. Clin Oncol (R Coll Radiol) 2015;27:307-15. Crossref
2. Schefter TE, Kavanagh BD, Timmerman RD, Cardenes HR, Baron A, Gaspar LE. A phase I trial of stereotactic body
radiation therapy (SBRT) for liver metastases. Int J Radiat
Oncol Biol Phys 2005;62:1371-8. Crossref
3. Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation
therapy for liver metastases. J Clin Oncol 2009;27:1572-8. Crossref
4. Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys
2010;76(3 Suppl):S94-100. Crossref
5. Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 2006;141:460-6. Crossref
6. Høyer M, Swaminath A, Bydder S, et al., Radiotherapy for liver metastases: a review of evidence. Int J Radiat Oncol
Biol Phys 2012;82:1047-57. Crossref
7. Prentice RL, Zhao S. Regression models and multivariate life tables. J Am Stat Assoc 2021;116:1330-45. Crossref
8. Hashemi R, Commenges D. Correction of the p-value after multiple tests in a Cox proportional hazard model.
Lifetime Data Anal 2002;8:335-48. Crossref
9. Rusthoven CG, Lauro CF, Kavanagh BD, Schefter TE. Stereotactic body radiation therapy (SBRT) for liver
metastases: a clinical review. Semin Colon Rectal Surg 2014;25:48-52. Crossref
10. Kobiela J, Spychalski P, Marvaso G, et al. Ablative stereotactic radiotherapy for oligometastatic colorectal
cancer: systematic review. Crit Rev Oncol Hematol 2018;129:91-101. Crossref
11. McPartlin A, Swaminath A, Wang R, et al. Long-term outcomes of phase 1 and 2 studies of SBRT for hepatic colorectal metastases. Int J Radiat Oncol Biol Phys 2017;99:388-95. Crossref
12. Scorsetti M, Comito T, Tozzi A, et al. Final results of a phase II trial for stereotactic body radiation therapy for patients with inoperable liver metastases from colorectal
cancer. J Cancer Res Clin Oncol 2015;141:543-53. Crossref
13. Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol 2006;45:823-30. Crossref
14. Joo JH, Park JH, Kim JC, et al. Local control outcomes using stereotactic body radiation therapy for liver metastases from colorectal cancer. Int J Radiat Oncol Biol Phys
2017;99:876-83. Crossref