© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Evaluation of contemporary olanzapine- and netupitant/palonosetron-containing antiemetic regimens for chemotherapy-induced nausea and vomiting
Christopher CH Yip1; L Li, MB, BS, FHKAM (Medicine)1; Thomas KH Lau, MB, BS, FHKAM (Medicine)1; Vicky TC Chan, MB, BS, FHKAM (Medicine)1; Carol CH Kwok, MB, BS, FHKAM (Radiology)2; Joyce JS Suen, MB, BS, FHKAM (Radiology)2; Frankie KF Mo, PhD1; Winnie Yeo, MB, BS, FHKAM (Medicine)1
1 Department of Clinical Oncology, Prince of Wales Hospital, Hong Kong
2 Department of Clinical Oncology, Princess Margaret Hospital, Hong Kong
Corresponding author: Prof Winnie Yeo (winnieyeo@cuhk.edu.hk)
Abstract
Introduction: This post-hoc analysis retrospectively
assessed data from two recent studies of antiemetic
regimens for chemotherapy-induced nausea and
vomiting (CINV). The primary objective was to
compare olanzapine-based versus netupitant/palonosetron (NEPA)-based regimens in terms of
controlling CINV during cycle 1 of doxorubicin/cyclophosphamide (AC) chemotherapy; secondary
objectives were to assess quality of life (QOL) and
emesis outcomes over four cycles of AC.
Methods: This study included 120 Chinese patients
with early-stage breast cancer who were receiving
AC; 60 patients received the olanzapine-based
antiemetic regimen, whereas 60 patients received the
NEPA-based antiemetic regimen. The olanzapine-based
regimen comprised aprepitant, ondansetron,
dexamethasone, and olanzapine; the NEPA-based
regimen comprised NEPA and dexamethasone.
Patient outcomes were compared in terms of emesis
control and QOL.
Results: During cycle 1 of AC, the olanzapine group
exhibited a higher rate of ‘no use of rescue therapy’
in the acute phase (olanzapine vs NEPA: 96.7% vs
85.0%, P=0.0225). No parameters differed between
groups in the delayed phase. The olanzapine
group had significantly higher rates of ‘no use of rescue therapy’ (91.7% vs 76.7%, P=0.0244) and ‘no
significant nausea’ (91.7% vs 78.3%, P=0.0408) in
the overall phase. There were no differences in QOL
between groups. Multiple cycle assessment revealed
that the NEPA group had higher rates of total control
in the acute phase (cycles 2 and 4) and the overall
phase (cycles 3 and 4).
Conclusion: These results do not conclusively support the superiority of either regimen for patients
with breast cancer who are receiving AC.
New knowledge added by this study
- The olanzapine-based regimen (aprepitant, ondansetron, dexamethasone, and olanzapine) and the NEPA-based regimen (netupitant, palonosetron, and dexamethasone) demonstrated similar efficacies in terms of controlling chemotherapy-induced nausea and vomiting among patients with early-stage breast cancer.
- Quality of life did not significantly differ between patients receiving the olanzapine-based regimen and patients receiving the NEPA-based regimen.
- The available data suggest that olanzapine-containing antiemetic regimens can be used without aprepitant, particularly when seeking to reduce medical expenses.
- Antiemetic efficacy may potentially be enhanced if NEPA is administered in combination with dexamethasone and olanzapine as a four-drug antiemetic regimen.
Introduction
Patients with breast cancer receiving (neo)adjuvant treatment exhibit improved prognoses.1 However,
chemotherapy regimens for breast cancer are
associated with various degrees of chemotherapy-induced nausea and vomiting (CINV). The
doxorubicin/cyclophosphamide (AC) regimen is
one of the most frequently prescribed regimens for
patients with breast cancer who are receiving (neo)
adjuvant chemotherapy; AC is among the highly emetogenic chemotherapies with ≥90% risk of nausea and vomiting.
In situations where a neurokinin-1 receptor
antagonist (NK1RA) is accessible, most current
guidelines for AC(-like) chemotherapy recommend
the use of a prophylactic triplet antiemetic regimen
that consists of an NK1RA, a 5-hydroxytryptamine
type-3 receptor antagonist (5HT3RA), and a
corticosteroid, with or without olanzapine.2 3 4
In addition to earlier NK1RAs (eg, aprepitant,
fosaprepitant, and rolapitant), netupitant/palonosetron (NEPA) [Akynzeo], which is a
combination of an NK1RA (netupitant 100 mg) and
a second-generation 5HT3RA (palonosetron 0.5
mg), has been available in the past decade. Although
palonosetron constitutes a more potent 5HT3RA,5 it
also has synergistic interactions with netupitant that
include interference with 5HT3 receptor cross-talk
and enhancement of the netupitant-mediated effect
on NK1 receptor internalisation.6 7
In a recent systematic review and meta-analysis,
Yokoe et al8 compared different antiemetic regimens to assess their control of CINV in
patients receiving highly emetogenic chemotherapy
regimens. The authors arbitrarily defined the
‘conventional’ regimen as a three-drug regimen
that contained dexamethasone, a first- or second-generation secondgeneration
5HT3RA, and an earlier NK1RA
compound (aprepitant, fosaprepitant, or rolapitant);
they defined ‘new’ regimens as regimens that
contained NEPA or olanzapine. The results indicated
that, compared with conventional regimens, new
regimens containing NEPA were more effective
in terms of producing a complete response (ie,
absence of vomiting and no use of rescue therapy).
Additionally, Yokoe et al8 showed that olanzapine-containing
regimens were most effective in terms
of producing a complete response, particularly
when olanzapine was added to a triplet regimen
of an NK1RA, a 5HT3RA, and dexamethasone.
These findings were supported by the results of a
prospective randomised study published in 2020,
which directly compared an olanzapine-containing
four-drug regimen with a standard triplet antiemetic
regimen (consisting of aprepitant, ondansetron,
and dexamethasone) for the prevention of CINV in
patients receiving AC chemotherapy.9
Here, we conducted a post-hoc analysis through
retrospective assessment of individual patient
data from two previously reported prospective
antiemetic studies that involved Chinese patients
with breast cancer.9 10 We hypothesised that a four-drug
antiemetic regimen (consisting of an NK1RA,
a 5HT3RA, dexamethasone, and olanzapine) would
remain superior to a three-drug regimen (consisting
of an NK1RA, a 5HT3RA, and dexamethasone)
that included NEPA as a combination NK1RA
and 5HT3RA agent. The primary objective was
to compare the efficacies of olanzapine- and
NEPA-containing antiemetic regimens in terms of
controlling CINV during the first cycle of AC. The
secondary objectives were: (1) to assess quality of
life (QOL) outcomes in patients receiving these
treatments during the first cycle of AC, and (2) to
assess emesis control outcomes in patients receiving
these treatments over multiple cycles of AC.
Methods
Patients
This study constituted a post-hoc analysis of data from two recently reported prospective studies. The
first prospective study investigated emesis outcomes
in patients with breast cancer who received a
standard triplet antiemetic regimen (ie, aprepitant,
ondansetron, and dexamethasone) with or without
olanzapine9; after the first study, a second prospective
study was conducted to assess the antiemetic efficacy
of NEPA and dexamethasone.10 These studies were
conducted with institutional ethics approval and
were registered at ClinicalTrials.gov (NCT03386617
and NCT03079219, respectively). For the post-hoc
analysis, data were extracted from the first study
regarding patients who received an olanzapine plus
aprepitant-containing four-drug antiemetic regimen; data were extracted from the second study regarding
patients who received NEPA and dexamethasone.
These patients were categorised into the ‘olanzapine’
and ‘NEPA’ groups, respectively.
Inclusion criteria were similar for the two
studies. Specifically, patients were eligible if they were
women of Chinese ethnicity, were aged >18 years,
had early-stage breast cancer, and planned to
receive a regimen of (neo)adjuvant AC. All study
participants were required to read, understand,
and complete study questionnaires and diaries
in Chinese. Exclusion criteria included abnormal
bone marrow, renal, or hepatic functions; receipt or
planned receipt of radiation therapy to the abdomen
or pelvis within 7 days prior to initial administration
of study treatment; presence of grade 2 to 3 nausea,
as defined by the National Cancer Institute Common
Terminology Criteria for Adverse Events version
4.0,11 or vomiting within 24 hours prior to initial
administration of the study treatment; presence of
an active infection or any uncontrolled disease; a
history of illicit drugs, including marijuana or alcohol
abuse; mental incapacitation; and/or presence of
a clinically significant emotional or psychiatric
disorder. Written consent was provided by eligible
patients prior to enrolment in the studies.
Study treatment
Patients in the olanzapine group received olanzapine 10 mg, aprepitant 125 mg, dexamethasone 12 mg,
and ondansetron 8 mg before chemotherapy on
day 1; they also received ondansetron 8 mg 8 hours
after chemotherapy. Subsequently, they received
aprepitant 80 mg daily on days 2-3 and olanzapine
10 mg daily on days 2-5.
Patients in the NEPA group received one
capsule of NEPA (netupitant 300 mg/palonosetron
0.50 mg) with dexamethasone 12 mg before
chemotherapy on day 1. Subsequently, they received
dexamethasone 4 mg twice per day on days 2-3.
Study assessments
At the initiation of chemotherapy on day 1, individual
patients were provided a diary to record the date and
time of their symptoms of vomiting and nausea for
120 hours after the AC infusion; the use of any rescue
medication was also recorded. On days 2-6, patients
rated their symptoms of nausea for the previous
24 hours using a visual analogue scale (in which 0 mm
implied no nausea, whereas 100 mm implied nausea
that was ‘as bad as it could be’). Additionally, on day 1
(before infusion of AC) and day 6 (after completion of
the diary), patients completed the Functional Living
Index-Emesis (FLIE) questionnaire. A research
nurse/assistant called individual patients on days
2-6 to remind them to take the study medications,
complete the patient diary, and complete the FLIE
questionnaire.
Assessment of efficacy and safety
Antiemetic efficacy was measured across three
overlapping time periods. The ‘acute’ phase
comprised 0 to 24 hours from the infusion of AC;
the ‘delayed’ phase comprised 24 to 120 hours from
the infusion of AC; the ‘overall’ phase comprised 0 to
120 hours from the infusion of AC.
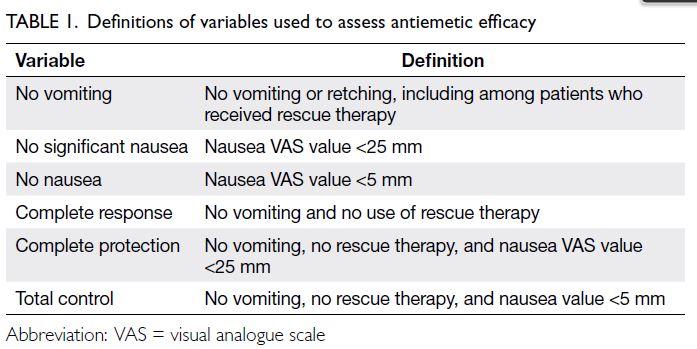
Variables used to assess antiemetic efficacy
were ‘complete response’, ‘no vomiting’, ‘no
significant nausea’, ‘no nausea’, ‘no use of rescue
therapy’, ‘complete protection’, and ‘total control’;
definitions of these variables are provided in Table 1. The proportions of patients who exhibited these
variables were recorded separately. Additionally, the
‘time to first vomiting’ in cycle 1 was determined
using information recorded in patients’ diaries.
Quality of life was evaluated using the Chinese
version of self-reported FLIE questionnaires from
individual patients.12 The FLIE questionnaire consists
of a nausea domain (9 items) and a vomiting domain
(9 items). All scores were transformed to ensure that
higher scores indicated worse impact on QOL.
Statistical analyses
A modified intention-to-treat approach was used
for all efficacy analyses; specifically, analyses
included patients who had received chemotherapy,
had completed the study procedures from 0 to 120
hours in cycle 1 of AC, and had no major protocol
violations.
To achieve the primary objective of this
study, the efficacies of the two antiemetic regimens
were based on the proportions (including 95%
confidence intervals) of patients who achieved
complete response during the acute, delayed, and
overall phases after AC infusion in cycle 1. Other
parameters compared in cycle 1 of AC were ‘time to
first vomiting’, ‘no vomiting’, ‘no significant nausea’,
no nausea’, ‘no use of rescue therapy’, ‘complete
protection’, and ‘total control’.
To achieve the secondary objectives, QOL was
compared between the two antiemetic regimens based on assessments of the nausea domain,
vomiting domain, and total score (sum of nausea
and vomiting domains) of the FLIE questionnaire
during cycle 1 of AC. Emesis control over multiple
cycles was compared between the two antiemetic
regimens by assessing the proportions (including
95% confidence intervals) of patients who achieved
‘complete response’, ‘complete protection’, and ‘total
control’ in the acute, delayed, and overall phases.
Comparisons between the two antiemetic
regimens were made using the Wilcoxon rank-sum
test for continuous data and Pearson’s Chi squared
test for dichotomous data. Two-sided P values <0.05
were considered statistically significant. The SAS Software version 9.4 (SAS Institute, Cary [NC], United States) was used for analyses.
Results
Patient characteristics
Data from 120 patients were included in this study; 60 patients each were enrolled in the NEPA and
olanzapine groups. Fifty-six patients (93.3%) in the
olanzapine group completed all four cycles of AC,
whereas 60 patients (100%) in the NEPA group
completed all four cycles of AC.
Patient characteristics, including
characteristics that could potentially affect CINV,
are shown in Table 2. The olanzapine and NEPA
groups had very similar patient characteristics, with
median ages of 54.5 and 56 years, respectively. Nearly
two-thirds of patients in each group had Stage II
breast cancer (63.3% and 66.7%, respectively). The
percentage of patients with a history of motion
sickness was higher in the NEPA group (35%) than
in the olanzapine group (16.7%). Furthermore, 30%
of patients in the NEPA group and 20% of patients
in the olanzapine group received AC as neoadjuvant
treatment.
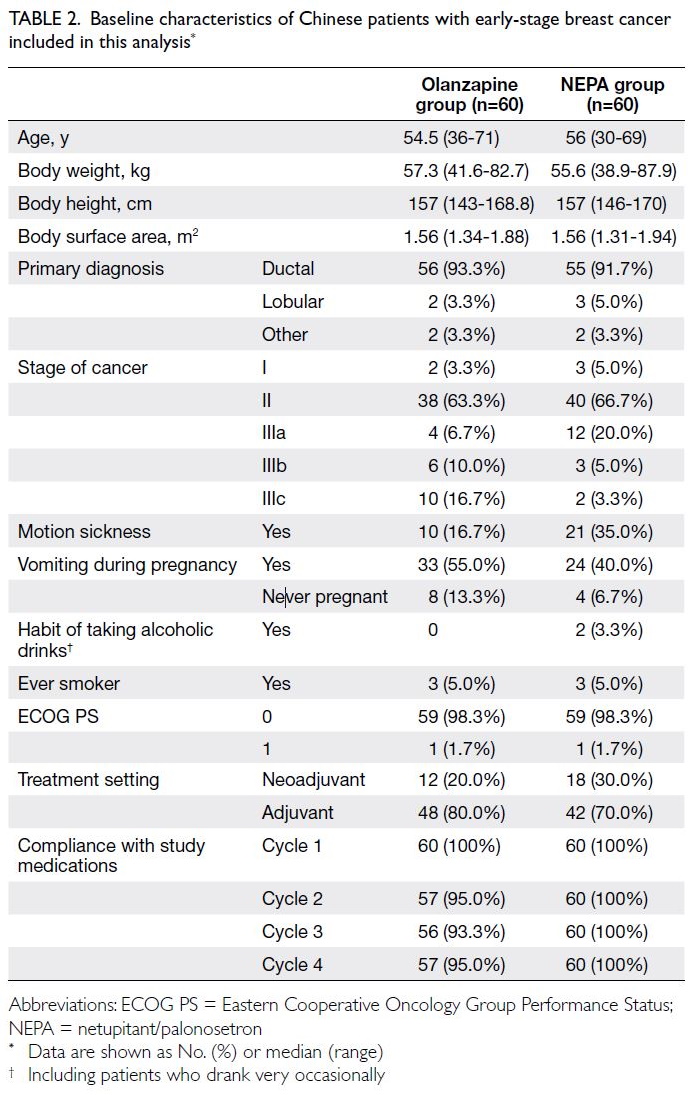
Table 2. Baseline characteristics of Chinese patients with early-stage breast cancer included in this analysis
Efficacy assessment
Antiemetic efficacies during cycle 1 of AC in the olanzapine and NEPA groups are shown in Table 3.
Complete response rates in acute, delayed, and
overall phases in cycle 1 did not differ between
groups. In the acute phase, the olanzapine group
exhibited a higher rate of ‘no use of rescue therapy’
(olanzapine vs NEPA: 96.7% vs 85.0%, P=0.0225). No
parameters differed between groups in the delayed
phase. In the overall phase, the olanzapine group
exhibited significantly higher rates of ‘no use of
rescue therapy’ (91.7% vs 76.7%, P=0.0244) and ‘no
significant nausea’ (91.7% vs 78.3%, P=0.0408).
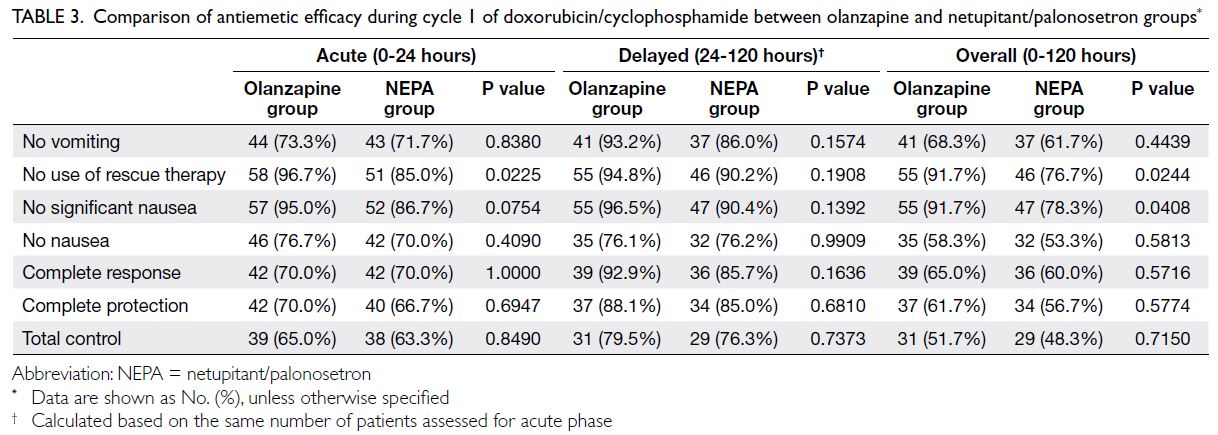
Table 3. Comparison of antiemetic efficacy during cycle 1 of doxorubicin/cyclophosphamide between olanzapine and netupitant/palonosetron groups
The median time to first vomiting was not
reached in either group (P=0.3902). Quality of
life results during cycle 1 of AC in the olanzapine
and NEPA groups, determined using the FLIE
questionnaire, are shown in the Figure. There were
no significant differences in the nausea domain,
vomiting domain, or total score of the FLIE
questionnaire between the two groups.
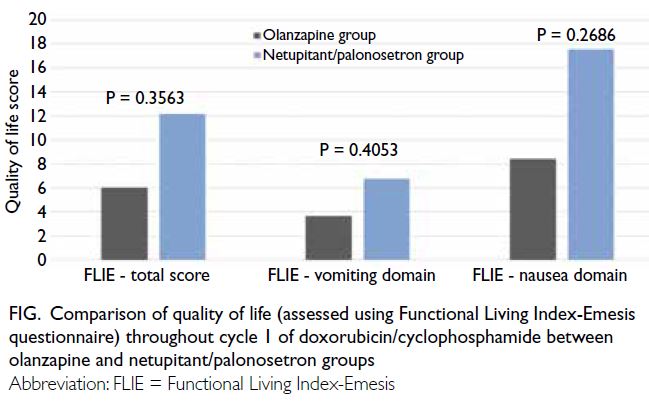
Figure. Comparison of quality of life (assessed using Functional Living Index-Emesis questionnaire) throughout cycle 1 of doxorubicin/cyclophosphamide between olanzapine and netupitant/palonosetron groups
Antiemetic efficacies over multiple cycles of
AC in the olanzapine and NEPA groups are shown
in Table 4. In the acute phase, the NEPA group
exhibited significantly higher rates of total control
in cycle 2 (olanzapine vs NEPA: 59.6% vs 81.7%,
P=0.0087) and cycle 4 (63.2% vs 86.7%, P=0.0032).
No parameters differed between groups in the
delayed phase. In the overall phase, the NEPA group
exhibited significantly higher rates of total control
in cycle 3 (55.4% vs 73.3%, P=0.0430) and cycle 4
(54.4% vs 75.0%, P=0.0195).
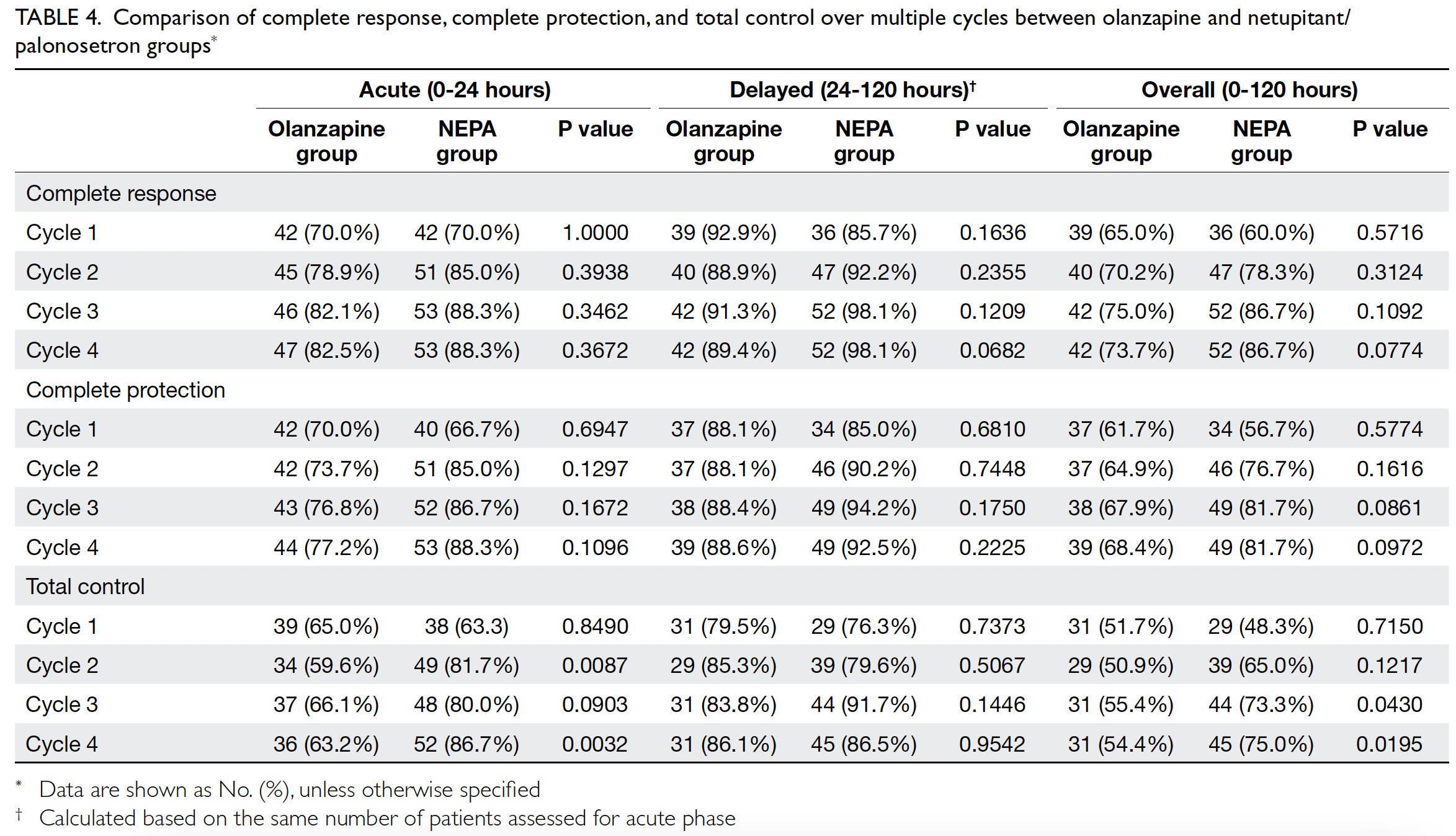
Table 4. Comparison of complete response, complete protection, and total control over multiple cycles between olanzapine and netupitant/palonosetron groups
Discussion
Chemotherapy-induced nausea and vomiting is
a frustrating adverse effect for patients receiving
anticancer treatment.13 The administration of
optimal antiemetic prophylaxis can help to
maintain QOL, while potentially improving patient
compliance in terms of completing planned
therapies. In current antiemetic prophylaxis
guidelines, the European Society of Medical
Oncology/Multinational Association of Supportive
Care in Cancer, the American Society of Clinical
Oncology, and the United States National
Comprehensive Cancer Network offer several
options regarding antiemetic regimens for patients
receiving AC(-like) chemotherapy. These options
mainly involve the combination of a 5HT3RA and
corticosteroids, with or without an NK1RA and
olanzapine.2 3 4 In particular, the incorporation of
olanzapine, an antipsychotic drug with antagonistic
effects on various receptors (eg, dopamine and
serotonin receptors),14 is increasingly regarded as a
component of antiemetic prophylaxis for patients
receiving anticancer treatment.
In an attempt to identify the best antiemetic
regimen, Yokoe et al8 conducted a meta-analysis
of randomised trials that tested various antiemetic
regimens. The results indicated that olanzapine-based
regimens demonstrated the best efficacy.
Specifically, olanzapine in combination with
an NK1RA, a 5HT3RA, and dexamethasone
exhibited the greatest efficacy; other olanzapine-containing
regimens (consisting of a 5HT3RA and
dexamethasone) were also superior to regimens that
lacked olanzapine. Moreover, even in the presence
of earlier NK1RAs (eg, aprepitant, fosaprepitant, or
rolapitant), regimens lacking olanzapine remained
inferior.
Similar to the findings with olanzapine, Yokoe
et al8 reported that triplet antiemetics involving NEPA were superior to conventional NK1RAs
(eg, aprepitant, fosaprepitant, or rolapitant).
Furthermore, Zhang et al15 directly compared NEPA-based
antiemetic regimens with aprepitant-based
triplet regimens in a randomised study that involved
800 patients who underwent administration of a
cisplatin-containing regimen. Their results revealed
that patients receiving NEPA and dexamethasone
exhibited similar control of CINV, compared with
patients receiving aprepitant, granisetron, and
dexamethasone; however, NEPA-treated patients
had a significantly lower requirement for rescue
therapy. Additionally, in a recent study focused on
patients with breast cancer who were undergoing
AC chemotherapy, patients who received NEPA and
dexamethasone demonstrated significantly higher
rates of complete response, complete protection,
and total control with enhanced QOL, compared
to historical controls who received aprepitant,
ondansetron, and dexamethasone; these benefits
persisted over multiple cycles of chemotherapy.10
To our knowledge, no study has directly
compared olanzapine- and NEPA-containing
regimens. Using an indirect comparison approach,
the present study showed that the olanzapine-based
regimen had higher rates of ‘no use of rescue
therapy’ and ‘no significant nausea’ in cycle 1 of AC,
compared to the NEPA-based regimen. In contrast,
assessments in subsequent cycles revealed that the
NEPA-based regimen led to higher rates of total
control in the acute phase (cycles 2 and 4) and the
overall phase (cycles 3 and 4). The lack of difference
in QOL between the two groups of patients may be
related to the difference in adverse-effect profiles of
the antiemetics used. For instance, the continued use
of dexamethasone on days 2-3 in the NEPA group may
have affected QOL among those patients because
of its effects on mood, insomnia, gastrointestinal
symptoms, and metabolic profiles.16 Indeed, a recent
meta-analysis showed that, among patients receiving
AC or moderately emetogenic chemotherapy, 3 days
of dexamethasone did not provide additional benefit
compared to 1 day of the agent.17 However, olanzapine
has been associated with sedation and somnolence.18
Thus, after the completion of a phase 2 trial in Japan
that suggested olanzapine was more effective at 5 mg
than at 10 mg,19 the same group of investigators
conducted a phase 3 study in which they tested the
addition of daily olanzapine 5 mg to an aprepitant-based
three-drug regimen; the results showed that,
even at a lower dose of olanzapine, the olanzapine-containing regimen remained more efficacious
than the olanzapine-free regimen for patients
receiving cisplatin.20 Other adverse effects have been
reported. Our analysis of olanzapine in combination
with aprepitant, ondansetron and dexamethasone
revealed a significantly higher incidence of grade
≥2 neutropenia in the olanzapine arm than in
the standard arm, although this altered incidence
was not associated with a significant difference
in neutropenic fever.9 A few cases of olanzapine-induced
neutropenia have been reported21;
additionally, a recent randomised antiemetic study
showed that patients who received an olanzapine-containing
regimen had a higher frequency of
severe neutropenia (without an increased incidence
of neutropenic fever).22 Although the underlying
mechanism remains unknown, the results of the
aforementioned Japanese study20 suggest that
olanzapine 5 mg could reduce the incidence of
neutropenia. In contrast, in our previous trial
regarding a NEPA-based regimen, we found that
patients in the NEPA arm had significantly lower
incidences of grade ≥2 neutropenia and neutropenic
fever, compared to historical controls who received
an aprepitant-based regimen.9 10
This study had some potential limitations.
First, dexamethasone was only used for 1 day in
the olanzapine-based regimen, whereas it was
administered for 3 days in the NEPA-based regimen;
this difference may have influenced the findings. Second, the use of data from two separate studies
may have affected the generalisability of the findings
because of slight variations in patient characteristics;
the lack of blinding in both studies also increased
the potential for patient-related reporting biases.
Nonetheless, the original studies were consecutively
conducted during the period from 2017 to 2019;
both the data from Chinese patients enrolled in a
homogenous group with early-stage breast cancer
who were receiving (neo)adjuvant AC chemotherapy
and the present analysis were analysed based on
individual patient data. These factors support the
validity of our comparison approach.
Conclusion
In conclusion, the present findings do not
conclusively support the superiority of either
the olanzapine-based regimen or the NEPA-based
regimen in terms of antiemetic efficacy or
QOL among patients with breast cancer who are
receiving AC. Our previous study demonstrated
that aprepitant has a limited effect when used with
a 5HT3RA and dexamethasone23; we also found
that NEPA was superior to aprepitant.10 Overall, the
available data suggest that olanzapine-containing
antiemetic regimens can be used without aprepitant,
particularly when seeking to reduce medical
expenses. Moreover, the available data support the
previous conclusion that, in parts of the world where
socio-economic limitations restrict the availability
of NK1RAs, the use of olanzapine combined
with a 5HT3RA and dexamethasone may be an
effective low-cost alternative antiemetic regimen.8 24
Antiemetic efficacy may be enhanced if NEPA is
administered in combination with dexamethasone
and olanzapine as a four-drug antiemetic regimen;
however, the efficacy of an olanzapine plus NEPA
regimen in terms of controlling CINV should be
confirmed in a trial setting.
Author contributions
Concept or design: W Yeo.
Acquisition of data: FKF Mo, W Yeo.
Analysis or interpretation of data: W Yeo, CCH Yip, FKF Mo.
Drafting of the manuscript: W Yeo, CCH Yip.
Critical revision of the manuscript for important intellectual content: L Li, TKH Lau, VTC Chan, CCH Kwok, JJS Suen, FKF Mo.
Acquisition of data: FKF Mo, W Yeo.
Analysis or interpretation of data: W Yeo, CCH Yip, FKF Mo.
Drafting of the manuscript: W Yeo, CCH Yip.
Critical revision of the manuscript for important intellectual content: L Li, TKH Lau, VTC Chan, CCH Kwok, JJS Suen, FKF Mo.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
W Yeo has been involved in the Chemotherapy-Induced Nausea and Vomiting (CINV) Network in Asia and
has provided lectures on CINV at events organised by
Mundipharma International Limited, which supported the
design of the NEPA study10 analysed in this post-hoc analysis but had no role in the present comparative analysis, data
collection and analysis, decision to publish, or preparation of
the manuscript.
Acknowledgement
We thank Ms Dong KT Lai, Ms Elizabeth Pang, Ms Vivian Chan, and Ms Maggie Cheung of the Department of Clinical
Oncology, The Chinese University of Hong Kong for their
support in contributing to patient enrolment and study
monitoring.
Declaration
Data from this study were presented at the European Society of Medical Oncology Asia Virtual Congress 2020 on 27
November 2020.
Funding/support
This study was supported by an education grant from Madam Diana Hon Fun Kong Donation for Cancer Research (Grant
No.: CUHK Project Code 7104870). The Donation had no
role in study design, data collection and analysis, decision to
publish, or preparation of the manuscript.
Ethics approval
The studies examined in this post-hoc analysis were approved
by The Joint Chinese University of Hong Kong–New
Territories East Cluster Institution Review Board of The
Chinese University of Hong Kong and the Hong Kong Hospital
Authority, and the Kowloon West Cluster Research Ethics
Committee of the Hong Kong Hospital Authority (Ref No.:
CREC 2016.013, CREC 2017.1609 and KW/FR-18-019[119-19]). All patient data in this study were anonymous and were
based on the abovementioned reported studies. There was no
additional work on retrieving patient records in this study.
References
1. Early Breast Cancer Trialists’ Collaborative Group
(EBCTCG); Peto R, Davies C, et al. Comparisons
between different polychemotherapy regimens for early
breast cancer: meta-analyses of long-term outcome
among 100,000 women in 123 randomised trials. Lancet
2012;379:432-44. Crossref
2. National Comprehensive Cancer Network. NCCN Clinical
Practice Guidelines in Oncology, Antiemesis Version 1; 2019.
3. Herrstedt J, Roila F, Warr D, et al. 2016 updated MASCC/ESMO consensus recommendations: prevention of nausea
and vomiting following high emetic risk chemotherapy.
Support Care Cancer 2017;25:277-88. Crossref
4. Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: American
Society of Clinical Oncology Clinical Practice Guideline
update. J Clin Oncol 2017;35:3240-61. Crossref
5. Popovic M, Warr DG, Deangelis C, et al. Efficacy and safety
of palonosetron for the prophylaxis of chemotherapy-induced
nausea and vomiting (CINV): a systematic review
and meta-analysis of randomized controlled trials. Support
Care Cancer 2014;22:1685-97. Crossref
6. Thomas AG, Stathis M, Rojas C, Slusher BS. Netupitant
and palonosetron trigger NK1 receptor internalization in
NG108-15 cells. Exp Brain Res 2014;232:2637-44. Crossref
7. Stathis M, Pietra C, Rojas C, Slusher BS. Inhibition of substance P-mediated responses in NG108-15 cells by netupitant and palonosetron exhibit synergistic effects.
Eur J Pharmacol 2012;689:25-30. Crossref
8. Yokoe T, Hayashida T, Nagayama A, et al. Effectiveness of
antiemetic regimens for highly emetogenic chemotherapy-induced
nausea and vomiting: a systematic review and
network meta-analysis. Oncologist 2019;24:e347-57. Crossref
9. Yeo W, Lau TK, Li L, et al. A randomized study of
olanzapine-containing versus standard antiemetic
regimens for the prevention of chemotherapy-induced
nausea and vomiting in Chinese breast cancer patients.
Breast 2020;50:30-8. Crossref
10. Yeo W, Lau TK, Kwok CC, et al. NEPA efficacy and tolerability during (neo)adjuvant breast cancer
chemotherapy with cyclophosphamide and doxorubicin.
BMJ Support Palliat Care 2022;12:e264-70.
11. National Cancer Institute. Cancer Therapy Evaluation
Program. 2021. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40. Accessed 3 Feb 2023.
12. Martin AR, Pearson JD, Cai B, Elmer M, Horgan K,
Lindley C. Assessing the impact of chemotherapy-induced
nausea and vomiting on patients’ daily lives: a modified
version of the Functional Living Index-Emesis (FLIE) with
5-day recall. Support Care Cancer 2003;11:522-7. Crossref
13. Griffin AM, Butow PN, Coates AS, et al. On the receiving
end. V: patient perceptions of the side effects of cancer
chemotherapy in 1993. Ann Oncol 1996;7:189-95. Crossref
14. Bymaster FP, Nelson DL, DeLapp NW, et al. Antagonism
by olanzapine of dopamine D1, serotonin2, muscarinic,
histamine H1 and alpha 1-adrenergic receptors in vitro.
Schizophr Res 1999;37:107-22. Crossref
15. Zhang L, Lu S, Feng J, et al. A randomized phase III
study evaluating the efficacy of single-dose NEPA,
a fixed antiemetic combination of netupitant and
palonosetron, versus an aprepitant regimen for prevention
of chemotherapy-induced nausea and vomiting (CINV)
in patients receiving highly emetogenic chemotherapy
(HEC). Ann Oncol 2018;29:452-8. Crossref
16. Roila F, Ruggeri B, Ballatori E, et al. Aprepitant versus metoclopramide, both combined with dexamethasone, for the prevention of cisplatin-induced delayed emesis: a
randomized, double-blind study. Ann Oncol 2015;26:1248-53. Crossref
17. Okada Y, Oba K, Furukawa N, et al. One-day versus three-day
dexamethasone in combination with palonosetron
for the prevention of chemotherapy-induced nausea and
vomiting: a systematic review and individual patient data-based
meta-analysis. Oncologist 2019;24:1593-600. Crossref
18. Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the
prevention of chemotherapy-induced nausea and vomiting.
N Engl J Med 2016;375:134-42. Crossref
19. Abe M, Hirashima Y, Kasamatsu Y, et al. Efficacy and safety
of olanzapine combined with aprepitant, palonosetron,
and dexamethasone for preventing nausea and vomiting
induced by cisplatin-based chemotherapy in gynecological
cancer: KCOG-G1301 phase II trial. Support Care Cancer
2016;24:675-82. Crossref
20. Hashimoto H, Abe M, Tokuyama O, et al. Olanzapine
5 mg plus standard antiemetic therapy for the prevention of
chemotherapy-induced nausea and vomiting (J-FORCE):
a multicentre, randomised, double-blind, placebo-controlled,
phase 3 trial. Lancet Oncol 2020;21:242-9. Crossref
21. Malhotra K, Vu P, Wang DH, Lai H, Faziola LR. Olanzapine-induced neutropenia. Ment Illn 2015;7:5871. Crossref
22. Gjafa E, Ng K, Grunewald T, et al. Neutropenic sepsis rates
in patients receiving bleomycin, etoposide and cisplatin
chemotherapy using olanzapine and reduced doses of
dexamethasone compared to a standard antiemetic
regimen. BJU Int 2021;127:205-11. Crossref
23. Yeo W, Mo FK, Suen JJ, et al. A randomized study
of aprepitant, ondansetron and dexamethasone for
chemotherapy-induced nausea and vomiting in Chinese
breast cancer patients receiving moderately emetogenic
chemotherapy. Breast Cancer Res Treat 2009;113:529-35. Crossref
24. Babu G, Saldanha SC, Kuntegowdanahalli
Chinnagiriyappa L, et al. The efficacy, safety, and
cost benefit of olanzapine versus aprepitant in highly
emetogenic chemotherapy: a pilot study from South India.
Chemother Res Pract 2016;2016:3439707. Crossref