Hong Kong Med J 2023 Feb;29(1):11-4 | Epub 8 Feb 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
EDITORIAL
The role of a single-shot higher-valency pneumococcal vaccine in overcoming challenges regarding invasive pneumococcal disease in Hong Kong
Christopher KM Hui, MB, BS, FRCP1; Ivan FN Hung, MD, PDiPID2,3; Bing Lam, MB, BS, PDipID4; Ada WC Lin , MB, BS, PDipID5; Thomas MK So, MB, BS, FRCP6; Andrew TY Wong, MB, BS, MPH7;
Martin CS Wong, MD, MPH8,9
1 813 Medical Centre, Hong Kong
2 Queen Mary Hospital, The University of Hong Kong, Hong Kong
3 Gleneagles Hospital Hong Kong, Hong Kong
4 Hong Kong Sanatorium & Hospital, Hong Kong
5 HKSH Medical Group, Hong Kong
6 Virtus Medical Centre, Hong Kong
7 Princess Margaret Hospital, Hong Kong
8 The Jockey Club School of Public Health and Primary Care, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong
9 Editor-in-Chief, Hong Kong Medical Journal
Corresponding author: Dr Christopher KM Hui (christopher.hui@uclmail.net)
Invasive pneumococcal disease (IPD), a major
public health problem worldwide (including in
Hong Kong),1 2 3 is a severe and potentially life-threatening
infectious disease caused by the gram-positive
bacterium, Streptococcus pneumoniae.1 2
The clinical manifestations of acute IPD vary among
organ systems involved; they include severe and
potentially fatal infections such as community-acquired
pneumonia, meningitis, and sepsis.2 In
Hong Kong, pneumonia has consistently been
the second leading cause of death since 20124; it
is associated with higher rates of hospitalisation
and higher healthcare costs, particularly among
older adults.5 6 Despite appropriate treatment,
up to 50% of IPD survivors experience long-term
complications, including respiratory, cardiovascular,
and neurological sequelae.7 Invasive pneumococcal
disease is associated with substantial healthcare
and economic burdens; thus, it represents an acute
public health problem in Hong Kong, particularly
amid the coronavirus disease 2019 (COVID-19)
pandemic. There is an urgent need to develop
effective strategies that can mitigate the potential
threat of an IPD outbreak.
Burden of invasive pneumococcal disease in
Hong Kong
Invasive pneumococcal disease has been a statutorily
notifiable disease in Hong Kong since January 2015.8
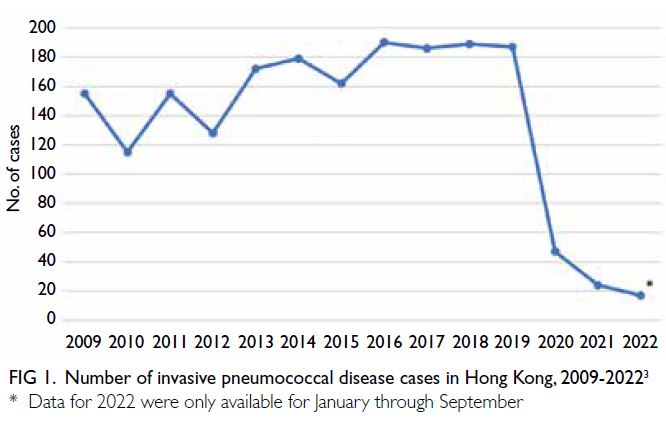
Between 2015 and 2019, the Centre for Health
Protection recorded a median of 187 (range: 162-190) IPD cases per year; the emergence of COVID-19 in 2020 led to a dramatic decrease in the number
of IPD cases in Hong Kong (Fig 1).3 However, the
current IPD burden is severely underestimated because of underdiagnosis, and a high index of
suspicion for IPD is a central aspect of differential
diagnosis. Because the clinical symptoms of IPD
overlap with the symptoms of other respiratory
illnesses, inexperienced physicians may experience
challenges regarding specimen collection (ie, samples
may be inappropriately or inadequately collected);
such challenges contribute to the underutilisation of
diagnostic tests and underreporting of IPD.
Because S pneumoniae is transmitted by direct
contact with respiratory secretions from patients
with IPD and from healthy carriers,2 9 public health
measures (eg, mask wearing, social distancing, travel
restrictions, and quarantine) that were implemented
to prevent the transmission of severe acute
respiratory syndrome coronavirus 2 also reduced
the spread of S pneumoniae; thus, the number of
IPD cases has decreased since the beginning of 2020
(Fig 1).3 As Hong Kong emerges from the COVID-19
pandemic, the gradual relaxation of public health
intervention measures is expected to result in
an increased number of IPD cases. Moreover,
seasonality could contribute to a sudden increase
in IPD cases because respiratory diseases (eg,
pneumococcal infection and influenza) are generally
more prevalent during winter and early spring.10 11
Notably, Israel experienced a nationwide outbreak
of S pneumoniae serotype 2 between 2015 and 2019,
despite the availability of vaccination programmes.12
Such outbreaks highlight the need to formulate
effective strategies for early disease prevention.
Pneumococcal vaccination in Hong Kong
Two types of pneumococcal vaccines are available to prevent IPD: pneumococcal polysaccharide vaccines (PPSVs) and pneumococcal conjugate vaccines
(PCVs). The 23-valent PPSV (PPSV23) contains
purified capsular polysaccharide antigens from 23
distinct S pneumoniae serotypes, whereas PCVs—including PCV13, PCV15, and PCV20—contain
purified capsular polysaccharide antigens from 13,
15 or 20 serotypes of S pneumoniae conjugated to
a nontoxic variant of diphtheria toxin (CRM197),
along with aluminium phosphate as an adjuvant.13 14
In contrast to PPSVs, the conjugated complexes
contained in PCVs exert long-term protection
because they are able to stimulate T-cell-dependent
immune response to generate immune memory for
the specific S pneumoniae serotypes covered by
the vaccine.15 Importantly, clinical trials and real-world
evidence have consistently demonstrated
the effectiveness of PCV13 in providing serotype-specific
protection against IPD.2 13 16 Although IPD
can occur at any age, an increased risk of onset
is associated with various factors; mortality is
substantially higher in children <2 years and adults
aged ≥65 years.10 13 In Hong Kong, the current
recommendations for pneumococcal vaccination
by the Centre for Health Protection prioritise adults
aged ≥65 years with high-risk conditions,17 consistent
with recommendations from the United States
Advisory Committee on Immunization Practices.18
Specifically, pneumococcal vaccine-naïve individuals
with high-risk conditions are recommended to
receive one dose of PCV13, followed by one dose of
PPSV23 at 1 year after PCV13 vaccination.17
Since 2017, the Hong Kong government
has provided free or subsidised pneumococcal
vaccination to eligible individuals through the
Government Vaccination Programme (GVP) and the
Vaccination Subsidy Scheme (VSS).19 Despite this
governmental support, rates of vaccine uptake and
participation in GVP and VSS remain low.19 Concerns
regarding vaccine efficacy, poor understanding of the disease, and lack of clarity regarding vaccine
schedules are some of the major challenges that
limit pneumococcal vaccination among adults in
Hong Kong.19 Another limiting factor is vaccine
hesitancy related to perceived vaccination burden
and fatigue.20
Current serotype burden in Hong Kong
Data from continuous surveillance of pneumococcal
serotypes have facilitated analyses of serotypes
isolated from the community, which have yielded
insights regarding the effectiveness and limitations
of pneumococcal vaccination programmes. Since
the implementation of pneumococcal vaccination in
Hong Kong, the incidence of IPD involving vaccine-covered
serotypes has considerably decreased.
However, because of low vaccination rates in
recent years, PCV13-covered serotypes (including
serotypes 3, 19F, and 19A) have been identified
in half of all reported IPD cases (Fig 2).3 21 22 23
Importantly, although it is covered by PCV13
and PPSV23, serotype 3 remains a major cause
of IPD because its unique polysaccharide capsule
resists detection by vaccine-induced antibodies.24
Moreover, the emergence of non-vaccine serotypes
(Fig 2; ie, serotype replacement) also poses a public
health threat.23 25
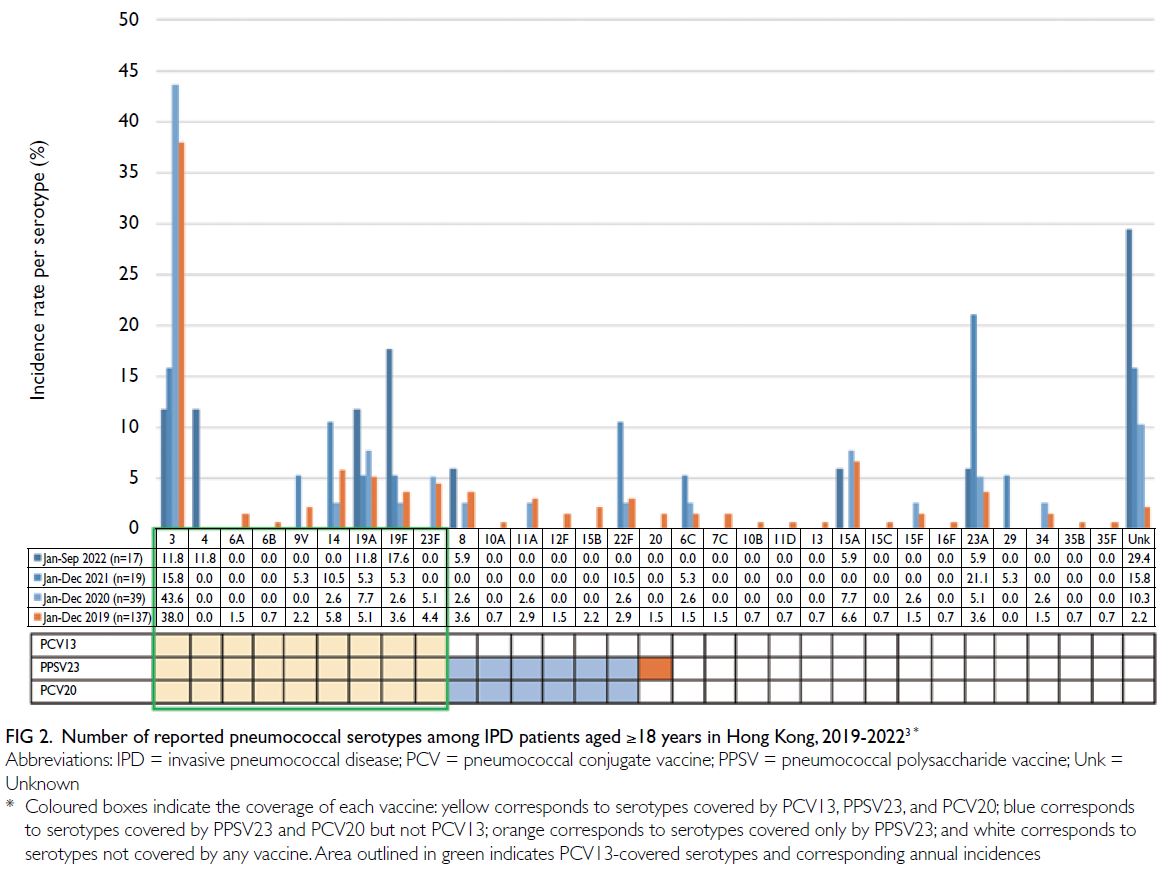
Figure 2. Number of reported pneumococcal serotypes among IPD patients aged ≥18 years in Hong Kong, 2019-20223
A higher-valency vaccine for broader
protection against invasive pneumococcal disease
Considering the current challenges in Hong Kong, a
higher-valency PCV (eg, PCV20) could partly address
the potential public health problem associated with
serotypes that are not covered by the Hong Kong
vaccination programme. The 20-valent PCV provides broader
protection against IPD; a single dose contains seven
new serotypes, in addition to the serotypes covered
by PCV13.26 Phase 3 studies of clinical efficacy
have demonstrated that PCV20 is noninferior to
PCV13 and PPSV23 across a subset of age-groups,
regardless of pneumococcal vaccination history and
high-risk conditions.27 28 Importantly, PCV20 can
be concurrently administered with influenza and
COVID-19 vaccines.26
In October 2021, the Advisory Committee on
Immunization Practices recommended one dose
of PCV20 alone, or serial immunisation (PCV15,
followed by PPSV23), for all PCV/PPSV-naïve
adults aged ≥65 years and PCV/PPSV-naïve adults
aged 19-64 years with high-risk conditions.26 The
implementation of a PCV20 single-shot vaccination
programme could be a cost-effective strategy
to address the current burden of IPD cases that
involve serotypes covered by PCV13 and serotypes
unique to PCV20.29 Furthermore, the convenience
of a simplified vaccination schedule could improve
vaccine uptake.
Overcoming challenges in Hong Kong and
implementing preventive strategies against invasive pneumococcal disease
The government and physicians play key roles
in promoting pneumococcal vaccination and
improving vaccine uptake, particularly among older
adults. Because the perceived low burden of IPD
may reduce the rate at which physicians recommend
vaccination for their patients,30 there is a need to
improve physician awareness regarding IPD and the
benefits of pneumococcal vaccines for individuals
with an increased risk of IPD.
Continuing medical education programmes
for physicians could cover periodic updates
regarding the IPD burden in Hong Kong, current
pneumococcal vaccine schedules, proper sample
collection methods, and appropriate diagnostic
tests for confirmation of IPD in patients with
relevant symptoms. These initiatives can improve
early diagnosis and treatment of IPD, facilitate
accurate data collection regarding IPD incidence,
and help to manage the underestimated burden of IPD. Additionally, government-led public education
campaigns that focus on bridging knowledge gaps
with respect to (i) the public health impact of IPD
(a vaccine-preventable disease), and (ii) vaccine
accessibility through GVP and VSS, could help to
overcome vaccine hesitancy and improve vaccine
uptake in Hong Kong.
Conflicts of interest
All authors declare no conflict of interest.
Author contributions
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Funding/support
Funding for this study was provided by Pfizer
Hong Kong. Editorial and medical writing support
was provided by Dr Analyn Lizaso from Weber
Shandwick Hong Kong, funded by Pfizer Hong Kong.
References
1. Wahl B, O’Brien KL, Greenbaum A, et al. Burden of
Streptococcus pneumoniae and Haemophilus influenzae
type b disease in children in the era of conjugate vaccines:
global, regional, and national estimates for 2000-15. Lancet
Glob Health 2018;6:e744-e757. Crossref
2. Weiser JN, Ferreira DM, Paton JC. Streptococcus
pneumoniae: transmission, colonization and invasion. Nat
Rev Microbiol 2018;16:355-67. Crossref
3. Centre for Health Protection, Department of Health,
Hong Kong SAR Government. Report on IPD. Available
from: https://www.chp.gov.hk/en/resources/29/636.html. Accessed 6 Aug 2022.
4. Centre for Health Protection. Death rates by leading causes of death, 2001-2021. Available from: https://www.chp.gov.hk/en/statistics/data/10/27/117.html. Accessed 12 Nov 2022.
5. Li X, Blais JE, Wong IC, et al. Population-based estimates of
the burden of pneumonia hospitalizations in Hong Kong,
2011-2015. Eur J Clin Microbiol Infect Dis 2019;38:553-61. Crossref
6. Man MY, Shum HP, Yu JS, Wu A, Yan WW. Burden of
pneumococcal disease: 8-year retrospective analysis
from a single centre in Hong Kong. Hong Kong Med J 2020;26:372-81. Crossref
7. Brooks LR, Mias GI. Streptococcus pneumoniae’s virulence
and host immunity: aging, diagnostics, and prevention.
Front Immunol 2018;9:1366. Crossref
8. Hong Kong e-Legislation. Cap. 599 Prevention and Control
of Disease Ordinance. Available from: https://www.elegislation.gov.hk/hk/cap599" target="_blank. Accessed Sep 2022.
9. Chan KP, Ma TF, Ip MS, Ho PL. Invasive pneumococcal
disease, pneumococcal pneumonia and all-cause
pneumonia in Hong Kong during the COVID-19 pandemic
compared with the preceding 5 years: a retrospective
observational study. BMJ Open 2021;11:e055575. Crossref
10. Centers for Disease Control and Prevention. Epidemiology
and Prevention of Vaccine-Preventable Diseases. 14th
edition. 2021. Available from: https://www.merle-arbeitsmedizin.de/wp-content/uploads/2022/02/CDC-Pink-Book-Version-14th-Edition.pdf. Accessed Nov 2022.
11. McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol ev
2006;19:571-82. Crossref
12. Dagan R, Ben-Shimol S, Benisty R, et al. A nationwide
outbreak of invasive pneumococcal disease in Israel caused
by Streptococcus pneumoniae serotype 2. Clin Infect Dis
2021;73:e3768-77. Crossref
13. Bridy-Pappas AE, Margolis MB, Center KJ, Isaacman DJ.
Streptococcus pneumoniae: description of the pathogen,
disease epidemiology, treatment, and prevention.
Pharmacotherapy 2005;25:1193-212. Crossref
14. Centers for Disease Control and Prevention. About pneumococcal vaccines. Available from: https://www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html. Accessed 12 Nov 2022.
15. Berger A. Science commentary: why conjugate vaccines protect longer. BMJ 1998;316:1571. Crossref
16. Theilacker C, Fletcher MA, Jodar L, Gessner BD. PCV13
vaccination of adults against pneumococcal disease:
what we have learned from the Community-Acquired
Pneumonia Immunization Trial in Adults (CAPiTA).
Microorganisms 2022;10:127. Crossref
17. Centre for Health Protection Scientific Committee on
Vaccine Preventable Diseases. Updated recommendations on the use of pneumococcal vaccines for high-risk
individuals. Available from: https://www.chp.gov.hk/files/pdf/updated_recommendations_on_the_use_of_pneumococcal_vaccines_for_high-risk_individuals.pdf.
Accessed 6 Oct 2022.
18. Morga A, Kimura T, Feng Q, Rozario N, Schwartz J. Compliance to Advisory Committee on Immunization
Practices recommendations for pneumococcal vaccination.
Vaccine 2022;40:2274-81. Crossref
19. Huang J, Mak FY, Wong YY, et al. Enabling factors, barriers,
and perceptions of pneumococcal vaccination strategy
implementation: a qualitative study. Vaccines (Basel)
2022;10:1164. Crossref
20. Su Z, Cheshmehzangi A, McDonnell D, da Veiga CP, Xiang YT. Mind the “Vaccine Fatigue”. Front Immunol
2022;13:839433. Crossref
21. Ho PL, Law PY, Chiu SS. Increase in incidence of invasive
pneumococcal disease caused by serotype 3 in children
eight years after the introduction of the pneumococcal
conjugate vaccine in Hong Kong. Hum Vaccin Immunother
2019;15:455-8. Crossref
22. Subramanian R, Liyanapathirana V, Barua N, et al.
Persistence of pneumococcal serotype 3 in adult
pneumococcal disease in Hong Kong. Vaccines (Basel)
2021;9:756. Crossref
23. Hon KL, Chan KH, Ko PL, et al. Change in pneumococcus
serotypes but not mortality or morbidity in pre- and
post-13-valent polysaccharide conjugate vaccine era:
epidemiology in a pediatric intensive care unit over 10
years. J Trop Pediatr 2018;64:403-8. Crossref
24. Luck JN, Tettelin H, Orihuela CJ. Sugar-coated killer:
serotype 3 pneumococcal disease. Front Cell Infect
Microbiol 2020;10:613287. Crossref
25. Lo SW, Gladstone RA, van Tonder AJ, et al. Pneumococcal
lineages associated with serotype replacement and
antibiotic resistance in childhood invasive pneumococcal
disease in the post-PCV13 era: an international wholegenome
sequencing study. Lancet Infect Dis 2019;19:759-69. Crossref
26. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent
pneumococcal conjugate vaccine among U.S. adults:
updated recommendations of the Advisory Committee on
Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep 2022;71:109-17. Crossref
27. Essink B, Sabharwal C, Cannon K, et al. Pivotal phase 3
randomized clinical trial of the safety, tolerability, and
immunogenicity of 20-valent pneumococcal conjugate
vaccine in adults aged ≥18 years. Clin Infect Dis 2022;75:390-8. Crossref
28. Cannon K, Elder C, Young M, et al. A trial to evaluate the
safety and immunogenicity of a 20-valent pneumococcal
conjugate vaccine in populations of adults ≥65 years of age
with different prior pneumococcal vaccination. Vaccine
2021;39:7494-502. Crossref
29. Mendes D, Averin A, Atwood M, et al. Cost-effectiveness
of using a 20-valent pneumococcal conjugate vaccine
to directly protect adults in England at elevated risk
of pneumococcal disease. Expert Rev Pharmacoecon
Outcomes Res 2022;22:1285-95. Crossref
30. Mui LW, Chan AY, Lee A, Lee J. Cross-sectional study
on attitudes among general practitioners towards
pneumococcal vaccination for middle-aged and elderly
population in Hong Kong. PLoS One 2013;8:e78210. Crossref