Hong Kong Med J 2022;28(6):447-56 | Epub 25 Nov 2022
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Paediatric high-grade osteosarcoma and its prognostic factors: a 10-year retrospective study
Grace PY Tong, MB, BS1; WF Hui, MB, ChB, MSc2; KC Wong, MB, ChB, MD (CUHK)3; Benjamin ST Fong, MB, BS4; CW Luk, MB, BS2; CK Li, MB, BS, MD (CUHK)5
1 Department of Paediatrics, Queen Elizabeth Hospital, Hong Kong
2 Department of Paediatrics, Hong Kong Children’s Hospital, Hong Kong
3 Department of Orthopaedics and Traumatology, Prince of Wales Hospital, Hong Kong
4 Department of Orthopaedics and Traumatology, Queen Elizabeth Hospital, Hong Kong
5 Department of Paediatrics, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong
Corresponding author: Dr Grace PY Tong (grace.tong@ha.org.hk)
Abstract
Introduction: This retrospective study was conducted to identify the characteristics of paediatric high-grade osteosarcoma and define its prognostic factors.
Methods: We identified paediatric patients (aged
<19 years at diagnosis) diagnosed with high-grade
osteosarcoma from 1 January 2009 to 31
December 2018 in two hospitals in Hong Kong, then
retrospectively evaluated their medical records to
identify prognostic factors.
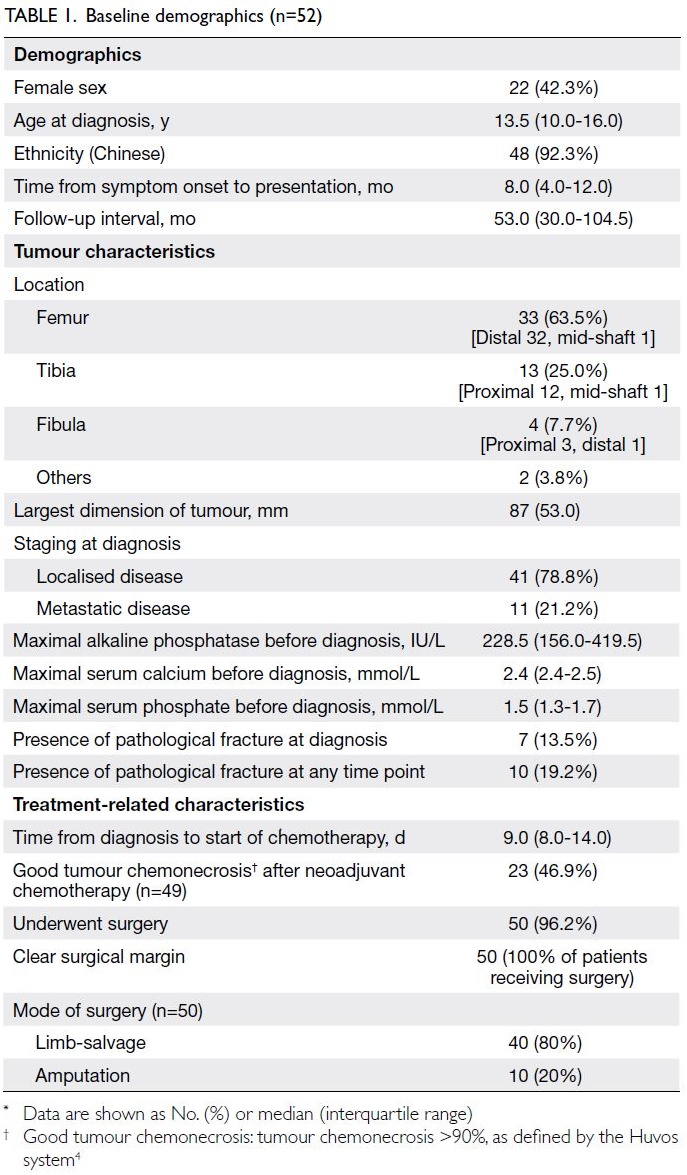
Results: In total, 52 patients were included in this
study (22 girls, 42.3%). Femoral tumour was the
most common form of osteosarcoma. Most patients
(78.8%) had localised disease at diagnosis. The lung
was the most common site of metastasis. Almost
half (n=23, 46.9%) of the patients showed a good
response to chemotherapy (ie, chemonecrosis >90%).
Most patients (n=40, 80%) underwent limb-salvage
surgery. The event-free survival and overall survival
rates were 55.8% and 71.2%, respectively. Prognostic
factors independently associated with poor event-free
survival and poor overall survival were the
presence of metastasis at diagnosis, poor tumour
chemonecrosis, and the need for amputation.
Conclusion: This multicentre review of paediatric high-grade osteosarcoma showed that the baseline
patient demographics, event-free survival, and
overall survival in Hong Kong were similar to previous
findings in other countries. Patients with metastatic
disease at diagnosis and poor chemonecrosis had
worse survival outcomes. Molecular analyses of
genetic abnormalities may help to identify targeted
therapies in future studies.
New knowledge added by this study
- The need for amputation was a prognostic factor independently associated with poor event-free survival and poor overall survival.
- Many conventional biochemical markers were not useful as prognostic factors for event-free survival or overall survival.
- An updated protocol is needed for the management of paediatric high-grade osteosarcoma. Factors that can be incorporated for early risk stratification include local tumour aggressiveness and the need for amputation; genetic mutations in the tumour may also be useful.
Introduction
Osteosarcoma arises from primitive bone-forming
mesenchymal cells.1 It is the most common primary
malignant bone tumour worldwide,1 2 with an annual
incidence of 4.8 per million population in the US.2
Moreover, it was one of the most common types
of childhood malignancy in Hong Kong in 2017 to
2019.3
In Hong Kong, paediatric patients with high-grade
osteosarcoma are treated in accordance
with the Hong Kong Paediatric Haematology and
Oncology Study Group Treatment Protocol for High-Grade Osteosarcoma (Fig 1), which consists
of neoadjuvant chemotherapy, followed by tumour
resection and subsequent adjuvant chemotherapy.
Histological response to neoadjuvant chemotherapy,
defined as tumour chemonecrosis according to
the Huvos system,4 is used to stratify patients into
good responders (patients with ≥90% tumour
chemonecrosis) and poor responders (patients with
<90% tumour chemonecrosis). These two groups
receive different chemotherapy regimens.
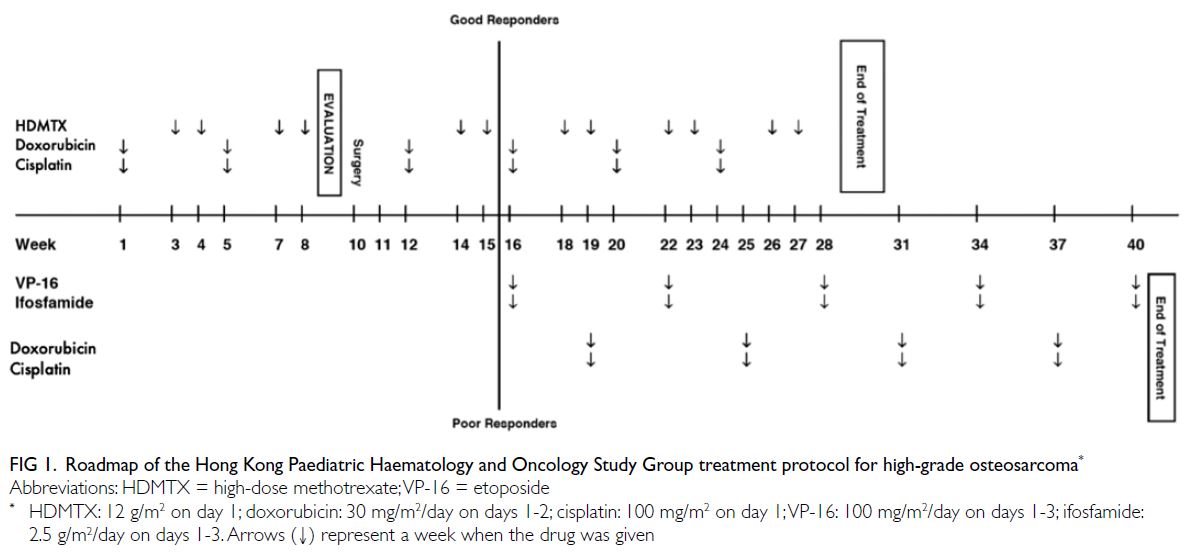
Figure 1. Roadmap of the Hong Kong Paediatric Haematology and Oncology Study Group treatment protocol for high-grade osteosarcoma
A few prognostic factors for survival have
been recognised; these factors include the presence of metastasis at diagnosis5 6 7 and poor tumour
chemonecrosis.5 6 7 8 9 However, no specific studies have
examined the validity of these factors for patients
with osteosarcoma in Hong Kong. Moreover, the
presence of lung nodules in computed tomography
(CT) scans is a common finding at diagnosis and
reassessment. In this study, we reviewed paediatric
patients with osteosarcoma at two of the largest
paediatric oncology centres in Hong Kong. We
sought prognostic factors for event-free survival
(EFS) and overall survival (OS), and we assessed lung nodules and metastasis in these patients.
Methods
This study included paediatric patients (aged
<19 years at diagnosis) with biopsy-proven high-grade
osteosarcoma, who were diagnosed from
1 January 2009 to 31 December 2018 at Queen
Elizabeth Hospital and Prince of Wales Hospital.
The patients’ medical records were reviewed and the
following information was collected: demographic
data (sex, age at diagnosis, ethnicity, and time
from symptom onset to presentation), clinical
characteristics (location of tumour, largest dimension
of tumour, staging, maximal alkaline phosphatase
[ALP] level, calcium and phosphate levels before
the start of treatment, and presence of pathological
fracture), treatment-related characteristics (time
from diagnosis to start of chemotherapy, surgical
treatment approach, and histological results of the
resected tumour [including tumour chemonecrosis
and surgical margin]), and details of metastasis
(timing, location, size, and treatment). Radiological
reports for the primary tumour were reviewed
to determine the presence of local anatomical
aggressiveness, which was defined as intra-articular
tumour involvement or neurovascular bundle
involvement. These risk factors made surgical
resection with a negative tumour margin difficult
or impossible. Thus, amputation was expected to
provide the best local control. There were three
indications for limb-salvage surgery (ie, tumour
resection and reconstruction). First, clinical and
radiological responses were observed during
neoadjuvant chemotherapy. Clinical responses were reduction or stabilisation of tumour size and a
significant decrease in pain. Radiological responses
were an increase in consolidated calcification
of the tumour on plain radiographs, reduction
or stabilisation of tumour size, and decreased
peritumoral oedema on magnetic resonance images.
Second, magnetic resonance images showed no
neurovascular involvement. Third, there was no need
for extensive muscle resection that would render the
limb non-functional. Amputation was considered
when patients did not meet the above criteria for
limb-salvage surgery. It was also considered as
a palliative treatment for patients who had large
painful tumours with metastatic disease.
The characteristics of lung nodules identified in
chest CT scans were recorded from CT reports. The
initial and final sizes, laterality, timing of appearance,
and mediastinal lymph node involvement were
analysed to determine whether their characteristics
were sufficiently different for clear distinction. Non-specific
lung nodules were either small or remained
stable in subsequent scans; they were not biopsied
or surgically excised for histological diagnosis. Lung
metastases were either large when first observed, had
radiological features of metastasis, or demonstrated
enlargement in subsequent follow-up scans.
Statistical analysis
Descriptive data were expressed as median
(interquartile range) or frequency (percentage). The
Pearson Chi squared test or Fisher’s exact test was
used for comparisons of categorical variables. The
Mann-Whitney U test was used for comparisons of
continuous variables.
The primary outcomes were OS and EFS.
Overall survival was defined as the time from
diagnosis to death. Event-free survival was defined
as the time from diagnosis to the appearance
of a new metastasis, progression of an existing
metastasis, or death (whichever occurred first). The
study end date was 31 December 2020. Patients who
had no events were censored at the time of the last
follow-up (if they had been lost to follow-up) or at
the study end date. Kaplan-Meier curves and log-rank
tests were used for survival analysis. To identify
prognostic factors for OS and EFS, unadjusted
hazard ratios (with 95% confidence intervals) were
determined for each potential factor by using Cox
proportional hazards models. Significant factors
in univariate analyses were included in subsequent
multivariate analyses; adjusted hazard ratios (with
95% confidence intervals) were generated in the
multivariate analyses.
The secondary outcome was the differentiation
of lung nodules. Their initial and final sizes, initial
and final lateralities, timing of appearance, and
mediastinal lymph node involvement were compared
to identify statistical differences.
The SPSS software (Windows version 23.0;
IBM Corp, Armonk [NY], US) was used for statistical
analysis. P values <0.05 were considered statistically
significant.
Results
Patient characteristics
In total, 52 paediatric patients (22 girls and 30 boys;
age 5-18 years) with high-grade osteosarcoma were
included in this study. Their baseline demographics
are shown in Table 1. Two patients (3.8%) had
a predisposing condition: one had Rothmund–Thomson syndrome and the other had osteofibrous dysplasia, from which the high-grade osteosarcoma
developed.
For the histological diagnosis, 45 patients
(86.5%) had conventional high-grade osteosarcomas.
The other subtypes of osteosarcoma were: two
giant cell-rich osteosarcomas, one telangiectatic
osteosarcoma, one chondrosarcomatous-predominant
osteosarcoma, one osteoblastoma-like
osteosarcoma, and one chondroblastic osteosarcoma.
One patient had features suggestive of small round-cell
sarcoma; the tumour was subsequently treated
as a conventional high-grade osteosarcoma.
Treatment
All patients received neoadjuvant chemotherapy with
doxorubicin, cisplatin, and high-dose methotrexate
(Fig 1). After two courses of chemotherapy, the
patients underwent surgical resection of tumours.
Almost all patients (n=50, 96.2%) underwent
resections of primary tumours. Most patients (n=44,
84.6%) completed the whole course of treatment
with adjuvant chemotherapy. Five patients (9.6%)
experienced disease progression during treatment. They had terminated chemotherapy early, received
palliative care, and eventually died. One patient
(1.9%) had disease progression and left Hong Kong
to seek a second opinion. In one patient (1.9%), the
last course of chemotherapy was omitted because
previous chemotherapy had induced clinically
significant renal impairment. In one patient (1.9%),
the last course of chemotherapy was omitted because
of disease progression while receiving treatment.
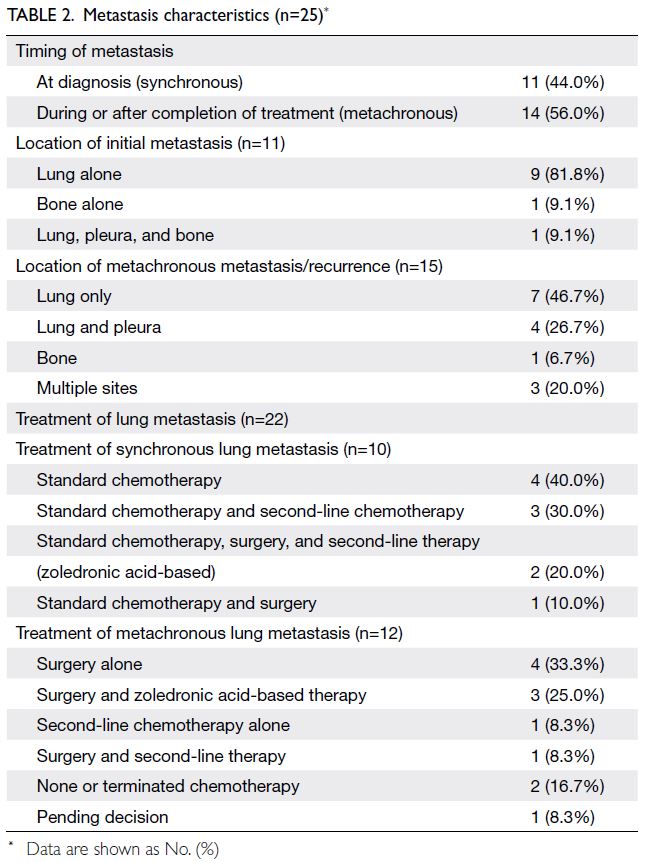
Metastasis and lung nodules
The lung was the most common site of distant
metastasis, both synchronous (n=10, 90.9%) and
metachronous (n=12, 80.0%) [Table 2]. Lung nodules
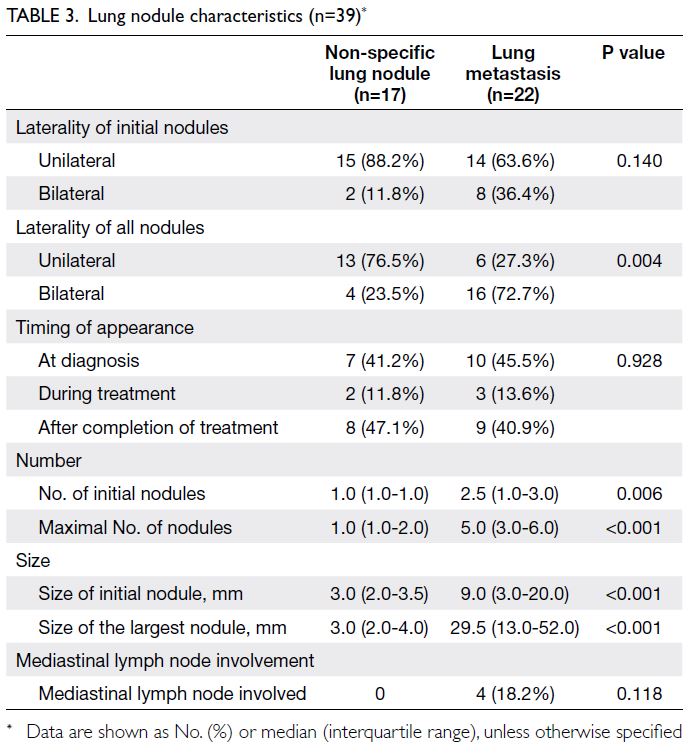
in chest CT scans were observed in 39 patients
(75.0%). Seventeen nodules (43.6%) were non-specific,
while 22 nodules (56.4%) were lung
metastases. Factors that could potentially be used
to differentiate lung metastases from non-specific
lung nodules included laterality of nodules in serial
follow-up scans (P=0.004), number of initial nodules
(P=0.006), maximal number of nodules (P<0.001),
size of initial nodule (P<0.001), and size of the largest
nodule (P<0.001) [Table 3].
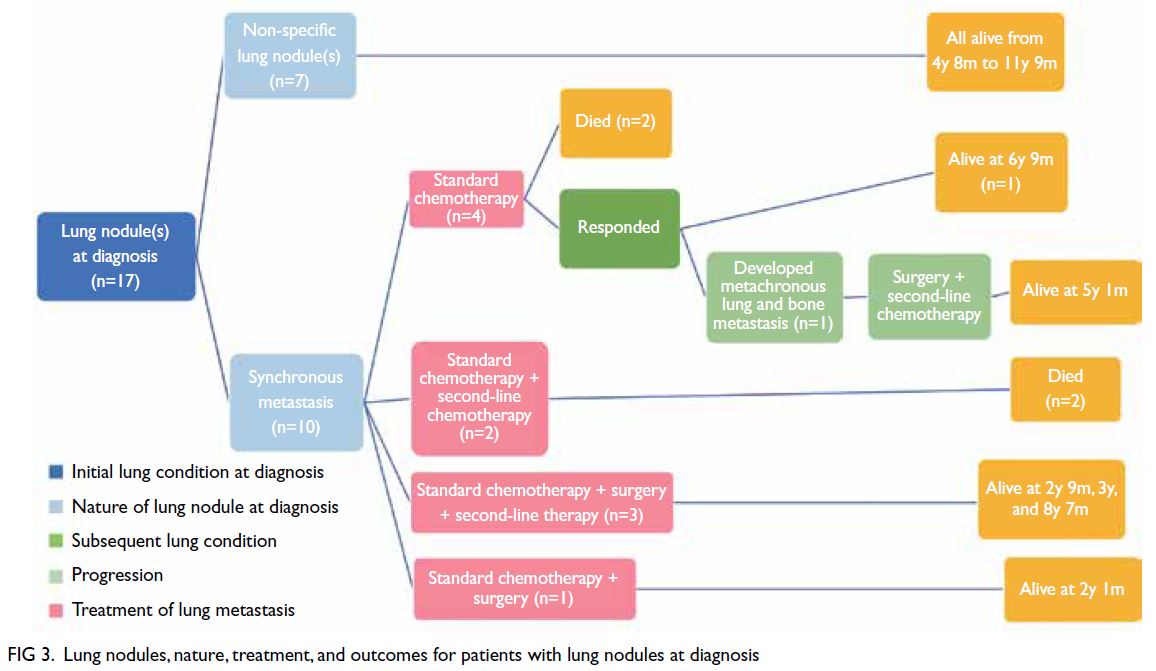
For the 15 patients who had no lung nodules
throughout the course of treatment, OS was very
good (93%) [Fig 2]. Only one patient died of local
recurrence. For the 17 patients with non-specific
lung nodules (Figs 2 and 3), OS was excellent (100%),
regardless of the timing of nodule appearance (ie,
at diagnosis or later). Among these 17 patients,
survival ranged from 2 years 4 months to 11 years
9 months from diagnosis. For the 10 patients with
synchronous lung metastasis (Fig 3), OS was 60%,
whereas for the 12 patients with metachronous lung
metastasis (Fig 2), OS was 33.3%. This difference was
not statistically significant (P=0.666).
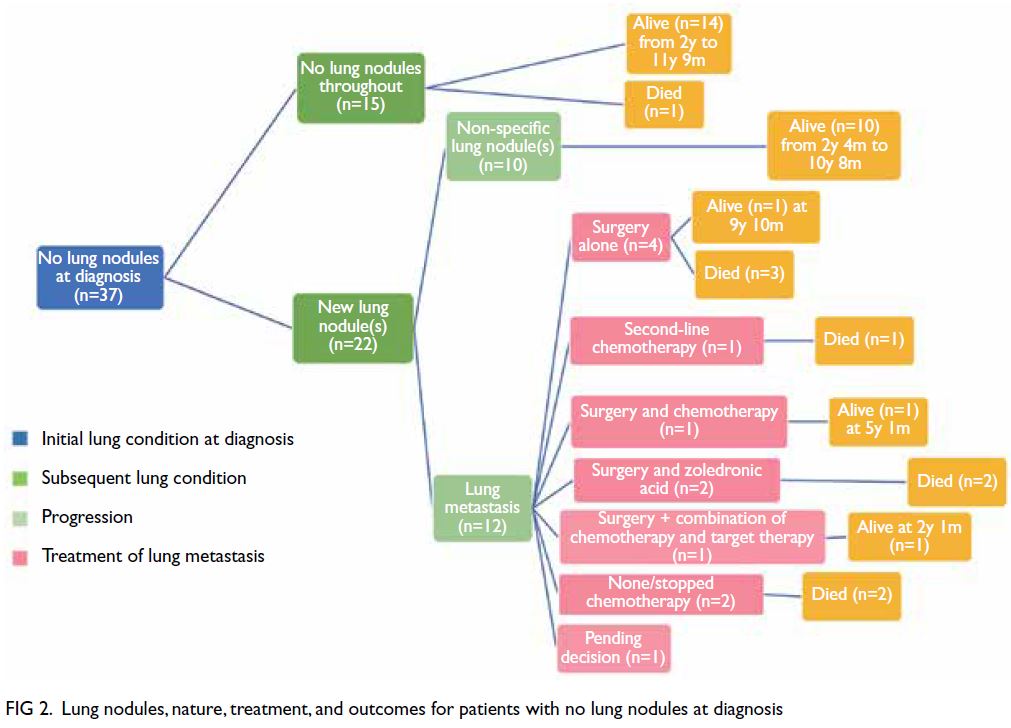
Figure 2. Lung nodules, nature, treatment, and outcomes for patients with no lung nodules at diagnosis
Survival analysis
All patients were followed up until 31 December
2020; thus, the follow-up interval for the last
recruited patient was 2 years from diagnosis. The
median follow-up interval for all patients was 53
months from diagnosis (range, 4 months to 11
years 11 months). The EFS and OS were 55.8%
and 71.2%, respectively. The EFS for patients with
localised disease at diagnosis was significantly better
than the EFS for patients with metastatic disease
at diagnosis (65.9% vs 18.2%; P=0.007). The OS
for patients with localised disease was better than
the OS for patients with metastatic disease (75.6%
vs 54.5%), but the difference was not statistically
significant (P=0.26). Similarly, patients with good
tumour chemonecrosis had better EFS and OS,
compared with poor responders. These values were
78.3% versus 42.3% (P=0.019) and 82.6% versus
65.4% (P=0.209), respectively. Notably, patients with
localised disease and good tumour chemonecrosis had very good outcomes, with EFS of 80% and OS
of 86.7%. All deaths in this study were caused by
disease progression. No patients died of treatment
complications or other causes.
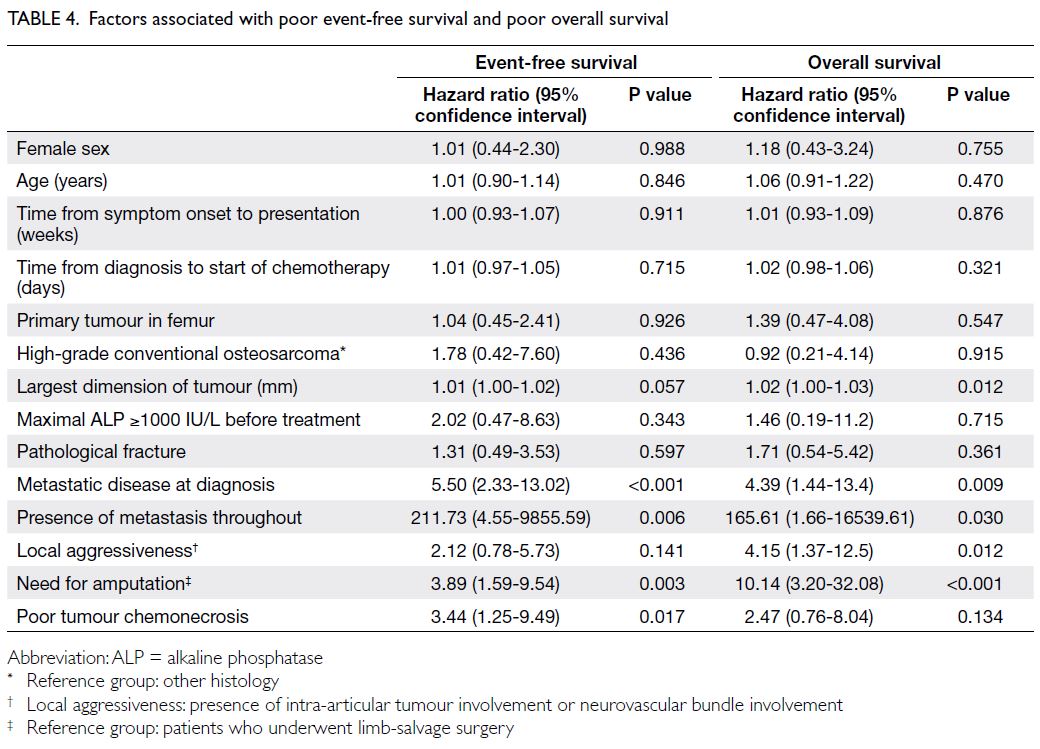
The prognostic factors identified for both
EFS and OS included the presence of metastasis at
diagnosis and throughout, as well as the need for
amputation to manage the primary tumour. Local
aggressiveness and a larger tumour dimension
contributed to OS, while poor tumour chemonecrosis
contributed to EFS (Table 4). The following factors
did not have a statistically significant impact on
EFS or OS: sex, age at diagnosis, primary tumour
location in the femur, the presence of a pathological
fracture, time from symptom onset to presentation,
time from diagnosis to start of chemotherapy, and
the histological subtype of the primary tumour.
Multivariate analysis (Table 5) revealed that
the presence of metastasis at diagnosis, the need
for amputation, and poor tumour chemonecrosis
were prognostic factors independently associated
with poor EFS, while the presence of metastasis
at diagnosis and the need for amputation were
prognostic factors independently associated with
poor OS. Figure 4 shows the Kaplan-Meier analysis
of the effects of the above three independent
prognostic factors on EFS.
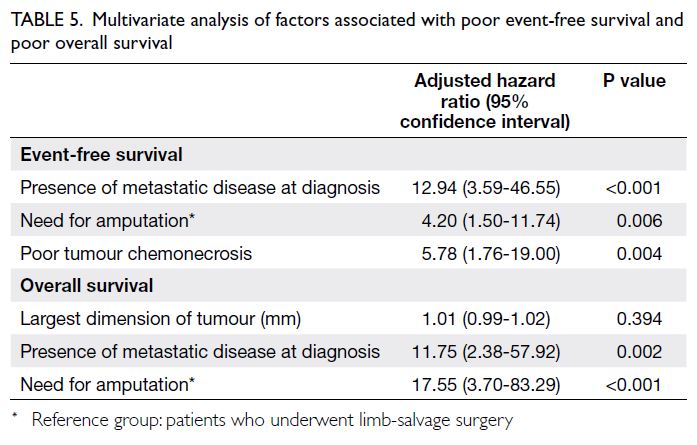
Table 5. Multivariate analysis of factors associated with poor event-free survival and poor overall survival
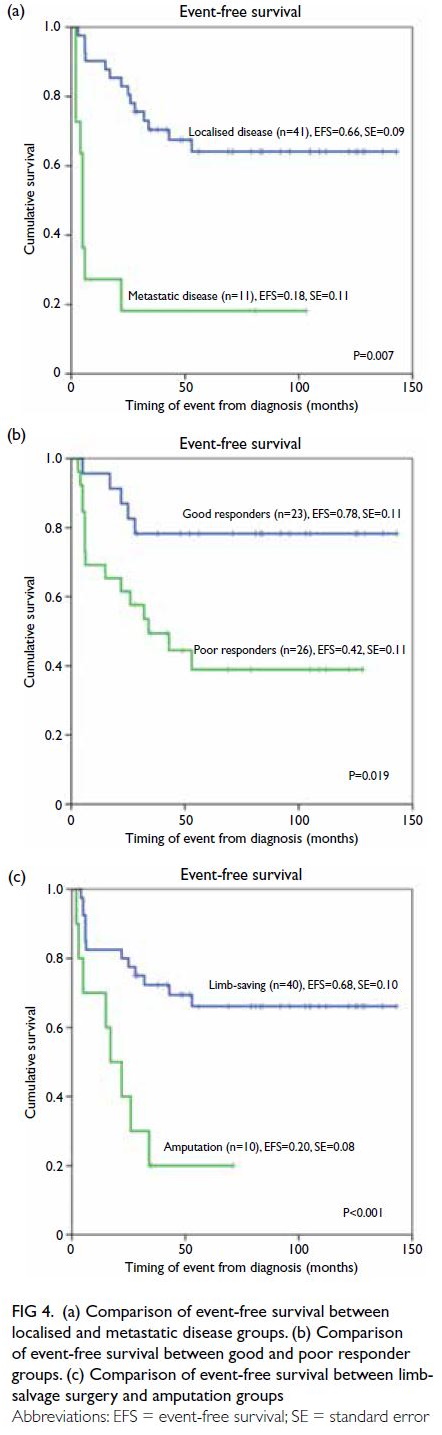
Figure 4. (a) Comparison of event-free survival between localised and metastatic disease groups. (b) Comparison of event-free survival between good and poor responder groups. (c) Comparison of event-free survival between limb-salvage surgery and amputation groups
Discussion
Patient characteristics
To our knowledge, this is the first multicentre review
of the demographic characteristics and prognostic
factors of paediatric high-grade osteosarcoma
in Hong Kong. The number of patients included
in the study constituted 76.5% of children with
osteosarcoma diagnosed in Hong Kong during
the study period (unpublished data). Thus, our
findings are likely to be representative of the
courses of disease and treatment for children with
osteosarcoma in Hong Kong. In this study, slightly
more patients with high-grade osteosarcoma were
boys, the median age at diagnosis was 13.5 years,
and the femur was the most common site of
involvement. All of these results are comparable to
findings in western countries.1 10 Only one patient
had a high-grade osteosarcoma in the humerus; no
patients had a primary nodule in the axial skeleton,
demonstrating the rarity of such nodules. Although
our study was limited by a short follow-up interval
in some patients, the EFS and OS were comparable
to the findings of large studies conducted in other
countries,6 8 10 11 as well as the results of a study
performed in Hong Kong in 2009.12 The shortest
follow-up interval was 2 years from diagnosis. Most
events occurred in the first 2 to 3 years, but some
poor responders experienced late relapse at 4 to
5 years after initial diagnosis. Thus, a longer follow-up
interval is necessary to better determine patient
outcomes and more comprehensively assess the
incidence of late toxicity.
Prognostic factors
In terms of prognostic factors, our findings in a
cohort of Hong Kong patients confirm the validity
of some important prognostic factors recognised
in studies performed elsewhere, including the
largest dimension of the primary tumour,8 13 14
presence of metastasis at diagnosis,6 8 9 11 and poor
tumour chemonecrosis.6 8 9 13 14 15 Additionally, our
study identified the need for amputation as an
independent prognostic factor for EFS and OS. With
respect to mortality, the prognostic effect of the
need for amputation has varied among studies.6 13 14 15 16
This variation is presumably because the decision
to amputate depends on many factors, including
the opinions of orthopaedic surgeons and parental
acceptance. In our study, 10 patients underwent
amputation; in two of these patients, the procedure
was performed with palliative intent to achieve
symptomatic control. Both of those patients had
metastatic disease at diagnosis, which involved large
and painful primary tumours. Thus, risk stratification
of patients according to the need for amputation may
enable the selection of patients with more advanced
disease. Nonetheless, in our cohort, all surgical margins were negative in both limb-salvage surgery
and amputation groups. Moreover, local recurrence
was uncommon. Thus, our findings may provide
insights that can be used to update risk stratification
protocols for paediatric patients with osteosarcoma.
In the current treatment protocol, there is a
considerable delay between initial diagnosis and risk
stratification (at week 16), which is performed after
tumour resection and when tumour chemonecrosis
data are available. However, the surgical approach
is usually determined after approximately 4 to 5
weeks of treatment, when reassessment imaging is
conducted. If aggressive features are observed at
diagnosis (eg, a massive tumour, early intra-articular
tumour involvement, or early neurovascular bundle
involvement), the discussion of possible amputation
may have already begun. Thus, the presence of such
features, or the early recognition of the need for
amputation, may be suitable for risk stratification
after validation in larger-scale studies.
Surrogate markers of aggressiveness
In recent decades, there has been extensive
research into surrogate markers for aggressiveness
in osteosarcoma. In some studies, the levels of
ALP8 9 14 15 17 and lactate dehydrogenase18 19 20 were
identified as significant prognostic factors. Although
the maximal ALP level was not associated with EFS
or OS in our study, some studies have demonstrated
a positive association between the serum ALP level
and tumour volume.21 This association is presumably
related to the increased rate of bone remodelling in
the tumour. However, the serum ALP level is also
elevated in teenagers because of increased bone
remodelling during periods of rapid growth. Thus,
a universal cut-off for all paediatric patients may not
provide the greatest prognostic accuracy.22 Some
studies23 have explored methods to increase the age specificity of serum ALP levels. However, more
validation and larger-scale studies are required before
these methods can be widely adopted. Whereas the
serum lactate dehydrogenase level showed a weaker
association with tumour volume,21 a change in this
level is more likely to be associated with a non-specific
increase in tumour metabolism. Currently,
the serum lactate dehydrogenase level is not included
in pretreatment staging and investigation protocols;
thus, it is not routinely checked. For investigation
purposes, it should also be included in baseline
investigations.
Because most biochemical parameters are not
accurate surrogate markers for the aggressiveness
of osteosarcoma, technological advancements
have enabled molecular and genetic profiling of
osteosarcomas to become the focus of research in the
past 10 to 15 years.22 24 25 These studies may provide
insights concerning the ‘non-conforming’ behaviour
of certain tumours in our patients; examples
include locally aggressive primary tumours that
warrant amputation but demonstrate good tumour
chemonecrosis, or tumours that show disease
progression despite good tumour chemonecrosis.
The current literature suggests that paediatric
osteosarcoma is a heterogeneous disease,21 26 although
general knowledge of the disease remains incomplete.
Despite advancements in surgical techniques for
primary tumour resection,27 improvements in the
accuracy of staging imaging, and enhancements
of supportive care, further revisions are needed
concerning the medical treatment of paediatric
osteosarcoma. Thus, molecular and genetic studies
are essential and may facilitate further stratification
of patients with osteosarcoma into different risk
groups, which may require tailored treatment
regimens for better outcomes. Future studies may
also enable the identification of molecular nodules
for targeted therapy or immunotherapy, which
lead to considerable advances in the treatment of
osteosarcoma.
Lung nodule analysis
Lung nodules were common in the initial staging and
serial follow-up CT scans of our patients. The small
size of some nodules hindered characterisation.
They might represent benign lung pathologies.
However, they may also represent micro-metastases
which were responsive to chemotherapy. Thus, they
remained stable in size and number on subsequent
scans, suggesting that they persisted as scars.
Patients with non-specific lung nodules had an
excellent prognosis, with an OS rate of 100% in
our study. However, repeated scans are needed for
the follow-up and characterisation of such lung
nodules. Additionally, the appearance of any new
lung nodules on CT scans creates a considerable
psychological burden for patients and their families. Our study identified parameters that can help to
differentiate true lung metastases from non-specific
nodules, including the number of initial nodules,
maximal number of nodules, initial nodule size,
and largest nodule size. A study in Hong Kong in
201128 also identified number (≤5 vs >5), size, and
laterality of lung nodules as important prognostic
factors for survival, whereas a study in the US in
201129 found that survival was worse for central
lung metastases than for peripherally located lung
metastasis. Additional larger-scale risk stratification
studies are needed to clearly delineate the cut-offs
for size, number, laterality, and location of initial
lung nodules. The establishment of a scoring system
that considers parameters of lung nodules observed
in chest CT scans may enable prediction of the risk
of malignancy, thus improving early detection of
lung metastasis and reducing unnecessary anxiety
for patients and their families. Furthermore,
standardised reviews of CT scans will help to
ensure more uniform classification of lung nodules,
particularly when the nodules are small.
With respect to confirmed lung metastasis,
the situation becomes increasingly complicated.
For a single synchronous or metachronous lung
metastasis, metastasectomy was conducted
whenever surgically feasible because it has been
regarded as the primary treatment approach in
multiple studies.30 31 32 However, given the diversity
of treatments, there was no consensus regarding
treatment strategies for multiple metastases or
local recurrence. This is presumably because the
effects of different chemotherapy regimens and
targeted therapies remain under investigation.11 33 34 35 36
In our study, surgical resection of lung metastasis
whenever possible, together with zoledronic acid,
generally achieved durable clinical remission for
>5 years. Long-term randomised controlled trials of
different chemotherapy or targeted therapy regimens
should be conducted to determine the most cost-effective
regimens that improve survival for relapsed
paediatric patients with high-grade osteosarcoma.
Many centres in other nations are performing
karyotyping and genetic analysis of osteosarcoma
tumour cells to identify targetable mutations.11 24 37
However, these tests are not routinely conducted
for standard care in Hong Kong. Collaborations
with centres in other nations should be pursued
to facilitate the implementation of international
standards in Hong Kong.
Conclusion
To our knowledge, this represents the first
multicentre review of paediatric high-grade
osteosarcoma in Hong Kong. Important prognostic
factors, including metastatic disease at diagnosis and
poor tumour chemonecrosis, were validated. The
need for amputation may reflect local aggressiveness, which influences OS. Larger-scale studies of high-grade
osteosarcoma in paediatric patients should
be conducted over a longer period of time to
better understand the characteristics, patterns, and
prognostic factors.
Author contributions
Concept or design: GPY Tong, CK Li.
Acquisition of data: GPY Tong.
Analysis or interpretation of data: GPY Tong, WF Hui, CK Li.
Drafting of the manuscript: GPY Tong, WF Hui, KC Wong, CK Li.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: GPY Tong.
Analysis or interpretation of data: GPY Tong, WF Hui, CK Li.
Drafting of the manuscript: GPY Tong, WF Hui, KC Wong, CK Li.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the
study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Research Ethics Committee
(Kowloon Central/Kowloon East Cluster and New Territories
East Cluster), Hospital Authority Hong Kong (Ref: KC/KE-19-0301/ER-1, 2019.712). The requirement for patient informed consent was waived.
References
1. Ottaviani G, Norman J. The epidemiology of osteosarcoma.
In: Jaffe N, Bruland OS, Bielack S, editors. Pediatric and
Adolescent Osteosarcoma. Boston, MA: Springer; 2009:
3-13. Crossref
2. North American Association of Central Cancer Registries (NAACCR), 2021.
3. Hong Kong Cancer Registry. Hong Kong Cancer Statistics 2015-2019.
4. Huvos AG, Rosen G, Marcove RC. Primary osteogenic sarcoma: pathologic aspects in 20 patients after treatment
with chemotherapy en bloc resection, and prosthetic bone
replacement. Arch Pathol Lab Med 1977;101:14-8.
5. Hung GY, Yen HJ, Yen CC, et al. Experience of pediatric
osteosarcoma of the extremity at a single institution in
Taiwan: prognostic factors and impact on survival. Ann
Surg Oncol 2015;22:1080-7. Crossref
6. Pakos EE, Nearchou AD, Grimer RJ, et al. Prognostic
factors and outcomes for osteosarcoma: an international
collaboration. Eur J Cancer 2009;45:2367-75. Crossref
7. Abou Ali B, Salman M, Ghanem KM, et al. Clinical
prognostic factors and outcome in pediatric osteosarcoma:
effect of delay in local control and degree of necrosis
in a multidisciplinary setting in Lebanon. J Glob Oncol
2019;5:1-8. Crossref
8. Vasquez L, Tarrillo F, Oscanoa M, et al. Analysis of
prognostic factors in high-grade osteosarcoma of the extremities in children: a 15-year single-institution
experience. Front Oncol 2016;6:22. Crossref
9. Mialou V, Philip T, Kalifa C, et al. Metastatic osteosarcoma
at diagnosis: prognostic factors and long-term outcome—the French pediatric experience. Cancer 2005;104:1100-9. Crossref
10. Marina NM, Smeland S, Bielack SS, et al. Comparison of
MAPIE versus MAP in patients with a poor response to
preoperative chemotherapy for newly diagnosed highgrade
osteosarcoma (EURAMOS-1): an open-label,
international, randomised controlled trial. Lancet Oncol
2016;17:1396-408. Crossref
11. Gorlick R, Janeway K, Lessnick S, Randall RL, Marina N.
Children’s Oncology Group’s 2013 blueprint for research:
bone tumors. Pediatr Blood Cancer 2013;60:1009-15. Crossref
12. Yang JY, Cheng FW, Wong KC, et al. Initial presentation
and management of osteosarcoma, and its impact on
disease outcome. Hong Kong Med J 2009;15:434-9.
13. Xin S, Wei G. Prognostic factors in osteosarcoma: a study
level meta-analysis and systematic review of current
practice. J Bone Oncol 2020;21:100281. Crossref
14. Bramer JA, van Linge JH, Grimer RJ, Scholten RJ.
Prognostic factors in localized extremity osteosarcoma: a
systematic review. Eur J Surg Oncol 2009;35:1030-6. Crossref
15. Hu J, Zhang C, Zhu K, et al. Treatment-related prognostic
factors in managing osteosarcoma around the knee with
limb salvage surgery: a lesson from a long-term follow-up
study. Biomed Res Int 2019;2019:3215824. Crossref
16. Jauregui JJ, Nadarajah V, Munn J, et al. Limb salvage versus
amputation in conventional appendicular osteosarcoma: a
systematic review. Indian J Surg Oncol 2018;9:232-40. Crossref
17. Zamzam MA, Moussa EA, Ghoneimy AE, et al. Outcomes
and prognostic factors for non-metastatic osteosarcoma of
the extremity. SM J Pediatr 2017;2:1013.
18. González-Billalabeitia E, Hitt R, Fernández J, et al.
Pre-treatment serum lactate dehydrogenase level is an
important prognostic factor in high-grade extremity
osteosarcoma. Clin Transl Oncol 2009;11:479-83. Crossref
19. Fu Y, Lan T, Cai H, Lu A, Yu W. Meta-analysis of serum
lactate dehydrogenase and prognosis for osteosarcoma.
Medicine (Baltimore) 2018;97:e0741. Crossref
20. Chen J, Sun M, Hua Y, Cai Z. Prognostic significance of
serum lactate dehydrogenase level in osteosarcoma: a
meta-analysis. J Cancer Res Clin Oncol 2014;140:1205-10. Crossref
21. Lindsey BA, Markel JE, Kleinerman ES. Osteosarcoma
overview. Rheumatol Ther 2017;4:25-43. Crossref
22. Savitskaya YA, Rico-Martínez G, Linares-González LM,
et al. Serum tumor markers in pediatric osteosarcoma: a
summary review. Clin Sarcoma Res 2012;2:9. Crossref
23. Shimose S, Kubo T, Fujimori J, Furuta T, Ochi M. A novel
assessment method of serum alkaline phosphatase for the diagnosis of osteosarcoma in children and adolescents. J
Orthop Sci 2014;19:997-1003.Crossref
24. de Nigris F, Zanella L, Cacciatore F, et al. YY1 overexpression
is associated with poor prognosis and metastasis-free
survival in patients suffering osteosarcoma. BMC Cancer
2011;11:472. Crossref
25. Morrow JJ, Khanna C. Osteosarcoma genetics and
epigenetics: emerging biology and candidate therapies.
Crit Rev Oncog 2015;20:173-97. Crossref
26. Poos K, Smida J, Maugg D, et al. Genomic heterogeneity
of osteosarcoma–shift from single candidates to functional
modules. PLoS One 2015;10:e0123082. Crossref
27. Wong KC, Kumta SM. Use of computer navigation in orthopedic oncology. Curr Surg Rep 2014;2:47. Crossref
28. Rasalkar DD, Chu WC, Lee V, Paunipagar BK, Cheng FW, Li
CK. Pulmonary metastases in children with osteosarcoma:
characteristics and impact on patient survival. Pediatr
Radiol 2011;41:227-36. Crossref
29. Letourneau PA, Xiao L, Harting MT, et al. Location
of pulmonary metastasis in pediatric osteosarcoma is
predictive of outcome. J Pediatr Surg 2011;46:1333-7. Crossref
30. Daw NC, Chou AJ, Jaffe N, et al. Recurrent osteosarcoma
with a single pulmonary metastasis: a multi-institutional
review. Br J Cancer 2015;112:278-82. Crossref
31. Matsubara E, Mori T, Koga T, et al. Metastasectomy of
pulmonary metastases from osteosarcoma: prognostic
factors and indication for repeat metastasectomy. J Respir
Med 2015;2015:1-5. Crossref
32. de Bree E, Drositis I, Michelakis D, Mavroudis D. Resection
of pulmonary metastases in osteosarcoma. Is it justified?
Hellenic J Surg 2018;90:293-8. Crossref
33. Geller DS, Gorlick R. Osteosarcoma: a review of diagnosis,
management, and treatment strategies. Clin Adv Hematol
Oncol 2010;8:705-18.
34. Warwick AB, Malempati S, Krailo M, et al. Phase 2 trial
of pemetrexed in children and adolescents with refractory
solid tumors: a Children’s Oncology Group study. Pediatr
Blood Cancer 2013;60:237-41. Crossref
35. Jacobs S, Fox E, Krailo M, et al. Phase II trial of ixabepilone
administered daily for five days in children and young
adults with refractory solid tumors: a report from the
children’s oncology group. Clin Cancer Res 2010;16:750-4. Crossref
36. Geoerger B, Chisholm J, Le Deley MC, et al. Phase II study
of gemcitabine combined with oxaliplatin in relapsed or
refractory paediatric solid malignancies: an innovative
therapy for children with Cancer European Consortium
Study. Eur J Cancer 2011;47:230-8. Crossref
37. Saraf AJ, Fenger JM, Roberts RD. Osteosarcoma:
accelerating progress makes for a hopeful future. Front
Oncol 2018;8:4. Crossref