© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Long-term tumour-treating fields for glioblastoma and beyond disease progression: a case report
Peter YM Woo, MB, BS, FRCS1; TC Lam, MB, BS, FRCR2; Aya El Helali, MB, BCh, PhD2
1 Department of Neurosurgery, Kwong Wah Hospital, Hong Kong
2 Department of Clinical Oncology, The University of Hong Kong, Hong Kong
Corresponding author: Dr Peter YM Woo (wym307@ha.org.hk)
Case report
In May 2018, a 55-year-old Chinese man experienced
sudden headache, vomiting and generalised seizures.
On hospitalisation, he had a Glasgow Coma Score
of 13/15 and global aphasia. Gadolinium contrast-enhanced
magnetic resonance imaging revealed a left
middle temporal gyrus heterogeneously enhancing
intra-axial brain tumour with intratumoural haemorrhage (Fig 1a). A craniotomy for gross total
tumour resection was performed under general
anaesthesia 1 day after admission (Fig 1b). The
patient fully recovered his language ability and was
discharged from the hospital 3 days after surgery
with no focal neurological deficit. At discharge,
he had a Karnofsky Performance Score of 90 and
an Eastern Cooperative Oncology Group (ECOG) performance status of 1. The histopathological
diagnosis was glioblastoma (IDH-1 wildtype,
promoter MGMT unmethylated). Targeted nextgeneration
gene sequencing revealed the presence
of CDKN2A homozygous deletion and EGFR
amplification, molecular biomarkers associated with
a poorer prognosis.1
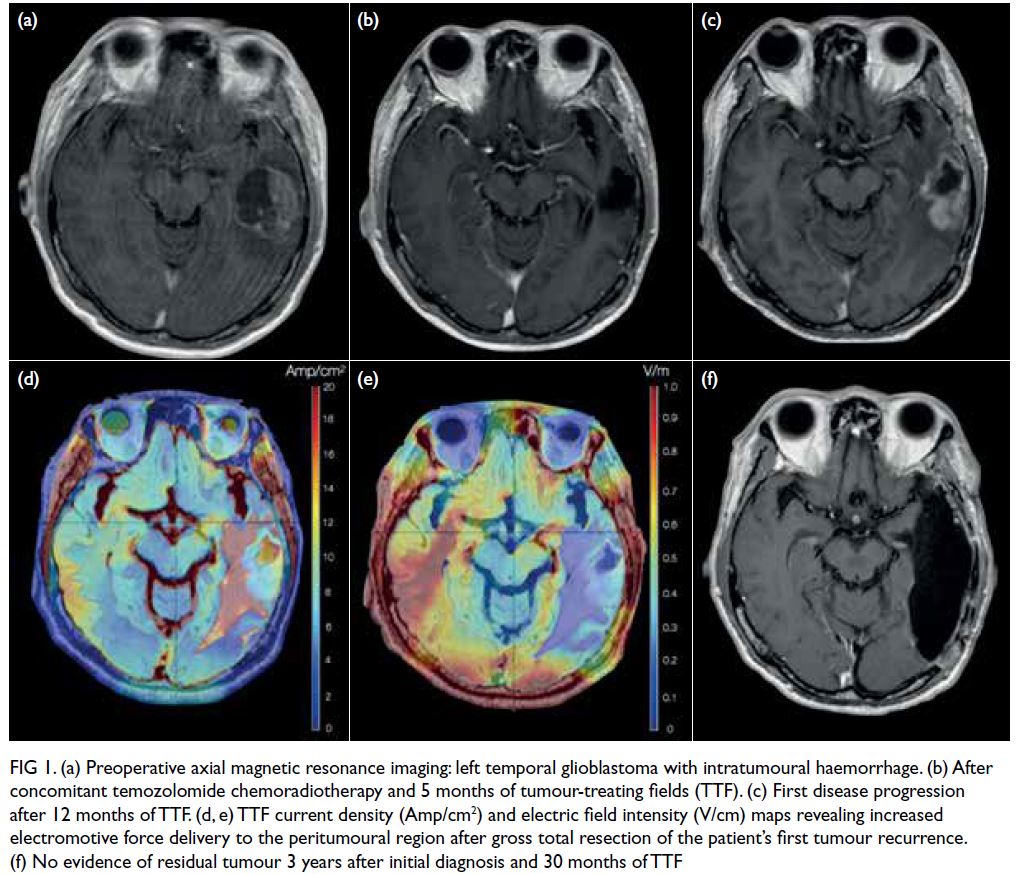
Figure 1. (a) Preoperative axial magnetic resonance imaging: left temporal glioblastoma with intratumoural haemorrhage. (b) After concomitant temozolomide chemoradiotherapy and 5 months of tumour-treating fields (TTF). (c) First disease progression after 12 months of TTF. (d, e) TTF current density (Amp/cm2) and electric field intensity (V/cm) maps revealing increased electromotive force delivery to the peritumoural region after gross total resection of the patient’s first tumour recurrence. (f) No evidence of residual tumour 3 years after initial diagnosis and 30 months of TTF
The patient received concomitant
temozolomide (TMZ) chemoradiotherapy with a
total of 60 Gy of radiation given over 30 fractions.
After three adjuvant cycles of TMZ, 6 months after
diagnosis, alternating electric field therapy also
known as tumour-treating fields (TTF) was started
in December 2018. After initiation, the patient
was able to return to work as a bartender with a
Karnofsky Performance Score of 100 and ECOG
status of 0. His mean monthly TTF compliance was
75% and although he experienced grade I scalp skin
toxicity (mild dermatitis), this was resolved with
topical hydrocortisone cream (Fig 2). The patient
received a total of six cycles of TMZ and declined
further chemotherapy, relying on TTF alone for
tumour control for the next 12 months. His EORTC
QLQ-C30 (European Organization for Research and
Treatment of Cancer global quality of life) score was
67/100 before TTF, which improved to 84/100 after
6 months. The caregiver stress index, a self-reported
measure of primary caregiver burden, was 2 (a
threshold of >7 indicates high stress).
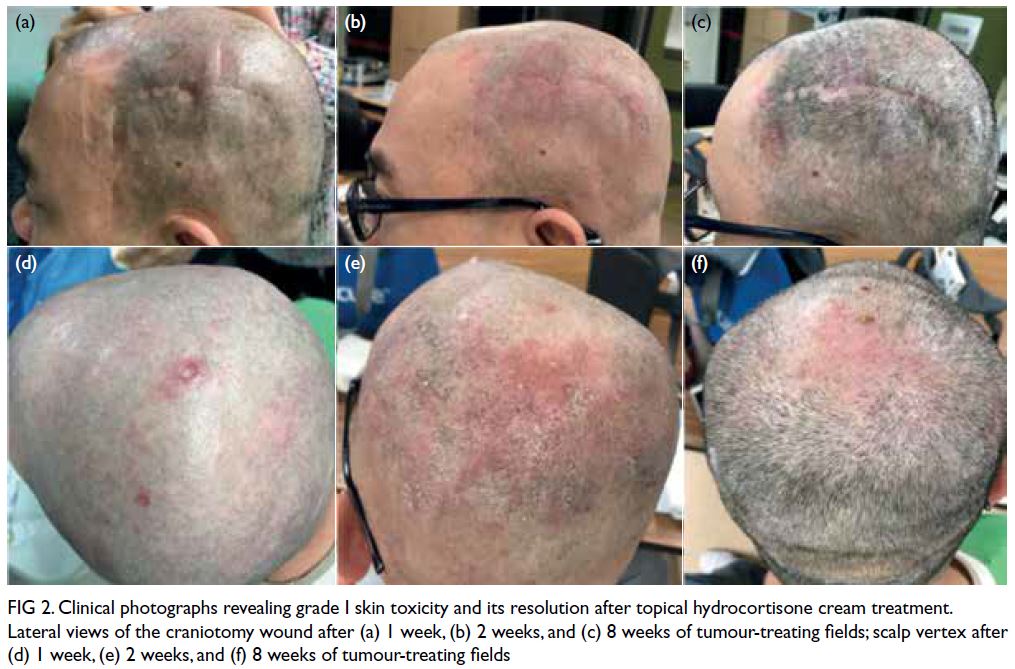
Figure 2. Clinical photographs revealing grade I skin toxicity and its resolution after topical hydrocortisone cream treatment. Lateral views of the craniotomy wound after (a) 1 week, (b) 2 weeks, and (c) 8 weeks of tumour-treating fields; scalp vertex after (d) 1 week, (e) 2 weeks, and (f) 8 weeks of tumour-treating fields
There was focal tumour recurrence 18 months after diagnosis and a second craniotomy with
supratotal resection was performed in December
2019 (Fig 1c). The patient received six cycles of
second-line lomustine chemotherapy and TTF
was restarted 6 weeks after the operation. The
treatment field plan was adjusted after the second
resection to enhance the current density (CD,
Amp/cm2) and electric field intensity (EF, V/cm)
to the peritumoural regions (Fig 1d and e). The
patient’s monthly TTF compliance increased to
85% and his ECOG status was 1. After 14 months,
in February 2021, there was a second glioblastoma
recurrence at the inferior temporal gyrus located
beyond the treatment EF50% and CD50% isodose
regions. An awake craniotomy for language mapping
and 5-aminolevulinic acid fluorescent-guided gross
total resection was performed. Since the patient’s
recurrent glioblastoma now had acquired TMZ and
lomustine resistance, without effective third-line
systemic therapy options, he was promptly restarted
on TTF 2 weeks after surgery achieving a mean
compliance of 90%. The patient received a further
10 months of TTF monotherapy after his second
recurrence, experiencing minimal adverse effects
with an ECOG performance status of 1, good quality
of life (EORTC 89/100) and no recurrence (Fig 1f). In
December 2021, multifocal disease progression with
leptomeningeal spread was detected and the patient
passed away in February 2022, 45 months (3.8 years)
after diagnosis.
Discussion
Glioblastoma is the most common primary malignant
brain tumour in adults with a prevalence of 3 to 5 per
100 000 population. In Hong Kong, 80 to 100 patients
are diagnosed annually. Multimodality standard-of-care
treatment has remained unchanged over the
last 15 years comprising of maximal safe resection
followed by concomitant TMZ chemoradiotherapy.2
Prognosis nonetheless remains poor and patients
have a median overall survival (OS) of only
15 months and for those with an unmethylated
pMGMT tumour molecular profile, 12 months.2
Tumour-treating fields is a novel therapy approved
by the United States Food and Drug Administration
and has been incorporated in several national
guidelines as a first-line treatment option for patients
with newly diagnosed glioblastoma. The therapy
consists of the non-invasive local administration of
alternating electric fields of low intensity (1-3 V/cm)
and intermediate frequency (200 kHz) to the post-resection
region. The mechanism of action involves
the exertion of an electromotive force on intracellular
proteins critical for mitosis, namely the microtubule
substrates tubulin and septin. The antimitotic effect
is most pronounced during the tumour cell cycle
metaphase when microtubule assembly is disrupted,
resulting in aneuploidy, post-mitotic stress, and
ultimately apoptosis.3
The efficacy of TTF in glioblastoma is well
supported by several randomised controlled trials.
The landmark EF-14 phase III trial that recruited
695 patients with newly diagnosed glioblastoma
revealed a significant increase in median OS
among those who received TTF and standard
TMZ chemoradiotherapy compared with their
control group counterparts that received standard
treatment alone (21 vs 17 months; hazard ratio=0.63;
95% confidence interval=0.53-0.76).3 The 2-year
OS rate for patients receiving TTF with standard
care was 43% compared with 29% for those that
received standard care alone.3 These findings were
independent of conventional predictors of OS
such as pMGMT methylation status or extent of
resection and were validated by subsequent studies.
Our patient’s glioblastoma carried a relatively
poor prognostic molecular profile and to observe
his longer-than-expected OS demonstrates how
TTF-generated antimitotic electromotive forces
remain unaffected by tumour chemoresistance
mechanisms. Studies have also documented a
dose-response relationship whereby mean monthly
treatment compliance, above a threshold of 60%,
was associated with improved median OS.3 4 5 This
phenomenon was also noted in our patient where his
first progression-free survival was 12 months with
75% TTF compliance but subsequently increased
to 14 months when his compliance was improved
to 85%. In general, the median OS of patients with recurrent glioblastoma is 6.5 months and it is
encouraging that our experience documented an
additional survival benefit from TTF beyond first
and second disease progression regardless of the
systemic therapy prescribed.6 7
The only modifiable predictor for OS is the
extent of glioblastoma resection8 and we believe this
played an important role in our patient’s response
to TTF. There is robust evidence that maximal
safe resection, even beyond radiologically defined
tumour boundaries (ie, supratotal resection),
confers a significant advantage.9 To this end, awake
craniotomy with intra-operative brain mapping and
5-aminolevulinic acid fluorescent guided resection
have been proven to be useful surgical adjuncts.10 11
In contrast, standards of care for systemic treatment
at recurrence are much less well-defined.7 12 Despite
limited evidence to support its use, lomustine, a
nitrosourea alkylating agent, is the most frequently
administered second-line treatment.12 Randomised
controlled clinical trials revealed lomustine
treatment response rates to only be in the range of
10%, conferring a median progression-free survival
of <2 months.12 13 Furthermore, lomustine activity is largely restricted to pMGMT methylated tumours,
which our patient did not have.12
Starting in December 2018, Hong Kong was
the first Asian region outside of Japan to provide
patients with access to TTF. Treatment is generally
started as early as 2 weeks after radiotherapy and
patients are required to have their hair clipped
during the entire period. The electric fields are
delivered through disposable adhesive scalp
transducer arrays connected to a portable generator
with interchangeable batteries, each lasting for
4 hours. Dosimetry in terms of field intensity (V/cm)
and current density (Amp/cm2) can significantly
influence OS therefore array positioning requires
an analysis of magnetic resonance imaging scans to
achieve the greatest therapeutic effect (Fig 1).4
Scalp arrays are typically changed every 3 days
when hair regrowth interrupts their apposition.
Patients are required to be constantly connected to
the 1.2-kg field generator for at least 15 hours per
day. Despite this treatment commitment, reviews of
the quality of life of TTF patients report outcomes
comparable to those without such therapy.14 The
most common adverse effect, occurring in up
to 45% of patients, is scalp dermatitis, which is
often mild to moderate in nature and sufficiently
managed by temporary array repositioning or
topical hydrocortisone.3 There is no evidence to
suggest that patients receiving TTF are at higher
risk of developing seizures. The only absolute
contra-indications to TTF are the presence of a large
skull defect, an active implantable medical device,
uncontrolled scalp wound infection, or allergies to
adhesive tape or hydrogels.
The TTF therapy is the first breakthrough
treatment for glioblastoma in >15 years. As exhibited
by our patient, long-term TTF therapy was well-tolerated
and conferred a significant benefit in terms of OS.
Author contributions
Concept or design: PYM Woo, TC Lam.
Acquisition of data: PYM Woo, TC Lam.
Analysis or interpretation of data: PYM Woo, TC Lam.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: PYM Woo, TC Lam.
Analysis or interpretation of data: PYM Woo, TC Lam.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Cluster (Ref No. UW
19-626). Patient consent available upon request.
References
1. Mirchia K, Richardson TE. Beyond IDH-mutation:
emerging molecular diagnostic and prognostic features in
adult diffuse gliomas. Cancers (Basel) 2020;12:1817. Crossref
2. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy
plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med 2005;352:987-96. Crossref
3. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating
fields plus maintenance temozolomide vs
maintenance temozolomide alone on survival in patients
with glioblastoma: a randomized clinical trial. JAMA
2017;318:2306-16. Crossref
4. Ballo MT, Urman N, Lavy-Shahaf G, Grewal J, Bomzon Z,
Toms S. Correlation of tumor treating fields dosimetry
to survival outcomes in newly diagnosed glioblastoma: a
large-scale numerical simulation-based analysis of data
from the phase 3 EF-14 randomized trial. Int J Radiat
Oncol Biol Phys 2019;104:1106-13. Crossref
5. Toms SA, Kim CY, Nicholas G, Ram Z. Increased
compliance with tumor treating fields therapy is prognostic
for improved survival in the treatment of glioblastoma: a
subgroup analysis of the EF-14 phase III trial. J Neurooncol
2019;141:467-73. Crossref
6. Kesari S, Ram Z, EF-14 Trial Investigators. Tumor-treating
fields plus chemotherapy versus chemotherapy alone for
glioblastoma at first recurrence: a post hoc analysis of the
EF-14 trial. CNS Oncol 2017;6:185-93. Crossref
7. van Linde ME, Brahm CG, de Witt Hamer PC, et al.
Treatment outcome of patients with recurrent glioblastoma
multiforme: a retrospective multicenter analysis. J
Neurooncol 2017;135:183-92. Crossref
8. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger
MS. An extent of resection threshold for newly diagnosed
glioblastomas. J Neurosurg 2011;115:3-8. Crossref
9. Jackson C, Choi J, Khalafallah AM, et al. A systematic
review and meta-analysis of supratotal versus gross total
resection for glioblastoma. J Neurooncol 2020;148:419-31. Crossref
10. Zhang JJ, Lee KS, Voisin MR, Hervey-Jumper SL, Berger MS,
Zadeh G. Awake craniotomy for resection of supratentorial
glioblastoma: a systematic review and meta-analysis.
Neurooncol Adv 2020;2:vdaa111. Crossref
11. Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided
surgery with 5-aminolevulinic acid for resection
of malignant glioma: a randomised controlled multicentre
phase III trial. Lancet Oncol 2006;7:392-401. Crossref
12. Weller M, Le Rhun E. How did lomustine become standard
of care in recurrent glioblastoma? Cancer Treat Rev
2020;87:102029. Crossref
13. Brada M, Stenning S, Gabe R, et al. Temozolomide versus
procarbazine, lomustine, and vincristine in recurrent high-grade
glioma. J Clin Oncol 2010;28:4601-8. Crossref
14. Taphoorn MJ, Dirven L, Kanner AA, et al. Influence
of treatment with tumor-treating fields on health-related
quality of life of patients with newly diagnosed
glioblastoma: a secondary analysis of a randomized clinical
trial. JAMA Oncol 2018;4:495-504. Crossref

