Hong Kong Med J 2022 Aug;28(4):294–9 | Epub 28 Jan 2022
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Effects of strict public health measures on
seroprevalence of anti–SARS-CoV-2 antibodies during pregnancy
Hillary HY Leung, MB, BS, BSc1; Christy YT Kwok, BMBS1; Daljit S Sahota, BEng, PhD1; Maran BW Leung, PhD1; Grace CY Lui, MB, ChB (Hons), PDipID2; Susanna SS N, g, MB, ChB, FHKAM (Medicine)3; WC Leung, MB, BS, MD (HKU)4; Paul KS Chan, MB, BS, MD (CUHK)2; Liona CY Poon, MB, BS, MD (Res) (University of London)1
1 Department of Obstetrics and Gynaecology, The Chinese University of Hong Kong, Hong Kong
2 Division of Infectious Diseases, Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong
3 Division of Respiratory Diseases, Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong
4 Department of Obstetrics and Gynaecology, Kwong Wah Hospital, Hong Kong
Corresponding author: (liona.poon@cuhk.edu.hk)
Abstract
Introduction: A substantial number of people
infected with severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) remain asymptomatic
throughout the course of infection. Nearly half of
pregnant women with coronavirus disease 2019
(COVID-19) are asymptomatic upon diagnosis; these
cases are not without risk of maternal morbidity.
Here, we investigated the seroprevalence of anti–SARS-CoV-2 antibodies in an unselected sample of
pregnant women in Hong Kong.
Methods: This prospective cohort study included
pregnant women who presented for routine Down
syndrome screening (DSS) between November 2019
and October 2020; all women subsequently delivered
at the booking hospitals. Serum antibodies against
SARS-CoV-2 were analysed using a qualitative
serological assay in paired serum samples taken at
DSS and delivery for all participants.
Results: In total, 1830 women were recruited.
Six women (0.33%) were seropositive at the DSS
visit; this seropositivity persisted until delivery. Of
the six women, none reported relevant symptoms
during pregnancy; one reported a travel history
before DSS and one reported relevant contact
history. The interval between sample collections
was 177 days (range, 161-195). Among women
with epidemiological risk factors, 1.79% with travel
history, 50% with relevant contact history, and 0.77%
with community SARS-CoV-2 testing history, were seropositive.
Conclusion: The low seroprevalence in this study
suggests that strict public health measures are
effective for preventing SARS-CoV-2 transmission.
However, these measures cannot be maintained
indefinitely. Until a highly effective therapeutic
drug targeting SARS-CoV-2 becomes available,
vaccination remains the best method to control the
COVID-19 pandemic.
New knowledge added by this study
- The seroprevalence of severe acute respiratory syndrome coronavirus 2 (anti–SARS-CoV-2) antibodies in an unselected sample of pregnant women in Hong Kong was low.
- Public health measures are effective for limiting the transmission of SARS-CoV-2.
- Anti–SARS-CoV-2 antibodies persist for at least 6 months.
- Serological testing could be utilised at antenatal screening to confirm the presence of anti–SARS-CoV-2 antibodies, preferably acquired through vaccination; such antibodies would provide some protection for the pregnant woman and her baby during the remaining portion of the pregnancy.
Introduction
A substantial number of people infected with severe
acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remain completely asymptomatic throughout
the course of infection.1 In a recent prospective observational study of maternal and neonatal
complications among 2130 pregnant women with
and without SARS-CoV-2 infection, 44% of pregnant
women diagnosed with coronavirus disease 2019
(COVID-19) were asymptomatic upon diagnosis. Despite this asymptomatic status, they exhibited
increased risks of maternal morbidity (relative
risk=1.24; 95% confidence interval=1.00-1.52) and
pre-eclampsia (relative risk=1.63; 95% confidence
interval=1.01-2.63), compared with pregnant women
who had not been diagnosed with COVID-19.2 In a
retrospective cohort study conducted in Spain, 3.1%
of 759 pregnant women exhibited anti–SARS-CoV-2
antibodies and had been asymptomatic throughout
pregnancy.3 Serological testing may serve as a useful
tool to identify pregnant women who have recovered
from a recent asymptomatic SARS-CoV-2 infection;
this information can help guide the management of
potential future complications.
Before the imposition of strict border control,
a large number of people travelled between Hong
Kong and the rest of the world each day. Pregnant
women could have been infected without their
knowledge because of asymptomatic or very mild
disease. Furthermore, pregnancy symptoms can
mask some COVID-19 symptoms, particularly if the
COVID-19 symptoms are mild.4 In this study, we
invited women who had undergone Down syndrome
screening (DSS) since November 2019 to ascertain
the seroprevalence of anti–SARS-CoV-2 antibodies
in an unselected sample of pregnant women in Hong
Kong. The findings were expected to provide insights concerning the asymptomatic infection rate among
pregnant women.
Methods
This prospective cohort study included pregnant
women who presented for routine DSS and
underwent routine blood sample collection at 11 to
13 weeks of gestation between November 2019 and
October 2020. All participants delivered at Kwong
Wah Hospital or Prince of Wales Hospital, Hong
Kong; the last delivery occurred in March 2021.
Eligibility criteria included consent to serum storage
for future research purposes and intention to deliver
at the booking hospital. Eligible women who attended
the booking hospital for delivery were invited to
participate in the study. Women who delivered
elsewhere, experienced pregnancy termination or
miscarriage, or received a diagnosis of COVID-19
before the study were excluded. Women who agreed
to participate in the study were asked to provide
written informed consent for blood collection at
delivery. Symptoms of COVID-19 throughout the
pregnancy were evaluated at recruitment and at
delivery.
Serum antibodies against SARS-CoV-2 were
analysed using a qualitative serological assay.
Qualitative detection of anti–SARS-CoV-2 antibodies
(immunoglobulin G [IgG] and immunoglobulin M
[IgM]) directed against the nucleocapsid protein
(N-protein) of the virus was performed using the
Elecsys Anti–SARS-CoV-2 assay (Roche, United
States) on a Cobas®e411 analyser. The result
was provided as a cut-off index (COI). A positive
anti–SARS-CoV-2 antibody result was defined as
COI >=1.0. Individuals who had recovered from
COVID-19 were recruited as positive control cases
(COI >=1.0); individuals who had negative SARS-CoV-2
test results were recruited as negative control cases
(COI <1.0) to ensure quality control in the anti–SARS-CoV-2 immunoassay.
Positive results based on qualitative detection
of anti–SARS-CoV-2 antibodies were subsequently
confirmed by quantitative measurements of IgG
and IgM antibodies against the SARS-CoV-2 spike
protein by using enzyme-linked immunosorbent
assays (ImmunoDiagnostics Limited, Hong Kong).
All tests were performed in duplicate, in accordance
with the manufacturer’s instructions. Results were
interpreted as negative when the optical density was
<0.2 and as positive when the optical density was
>=0.2. For samples with positive results, anti–spike
protein concentrations (ng/mL) were calculated.
Continuous variables were expressed
as medians (interquartile ranges or ranges).
Categorical variables were summarised as counts
and percentages. SPSS Statistics (Windows version
26.0; IBM Corp, Armonk [NY], United States) was
used for data analyses.
The STROBE reporting guidelines were used
during the preparation of this manuscript.
Results
In total, 3219 consecutive pregnant women were
approached; 306 declined participation and 1083
were excluded, including two with laboratory-confirmed
SARS-CoV-2 infection, 69 who
experienced miscarriage or pregnancy termination,
and 1012 who planned delivery elsewhere. Thus,
1830 women were recruited to the study and
provided written informed consent to participate.
In total, 1810 (98.9%) women were Chinese, and
852 (46.6%) women were nulliparous. The median
(interquartile range) maternal weight, height and
age were 55.5 kg (50.3-62.5), 159 cm (155-163) and
33.0 years (30.2-36.4), respectively.
In total, six women (0.33%) were seropositive
(COI >=1) at the DSS visit; this seropositivity persisted
until delivery. Among these six women, one exhibited
both anti–SARS-CoV-2 IgG and IgM antibodies;
the IgM antibodies were undetectable at delivery.
The remaining seropositive women exhibited only
anti–SARS-CoV-2 IgG antibodies at both visits. All
six of these women reported no relevant symptoms
during pregnancy. Among the six women, one
reported a travel history before her first DSS and one
reported relevant contact history. The median COIs were 2.465 (interquartile range=1.430-3.178) and
1.680 (interquartile range=1.145-2.350) at DSS and
delivery, respectively. The interval between sample
collections was 177 days (range, 161-195). The COI
at delivery was lower by a median of 31.8% (range,
12.0-35.1), compared with the COI at the DSS visit.
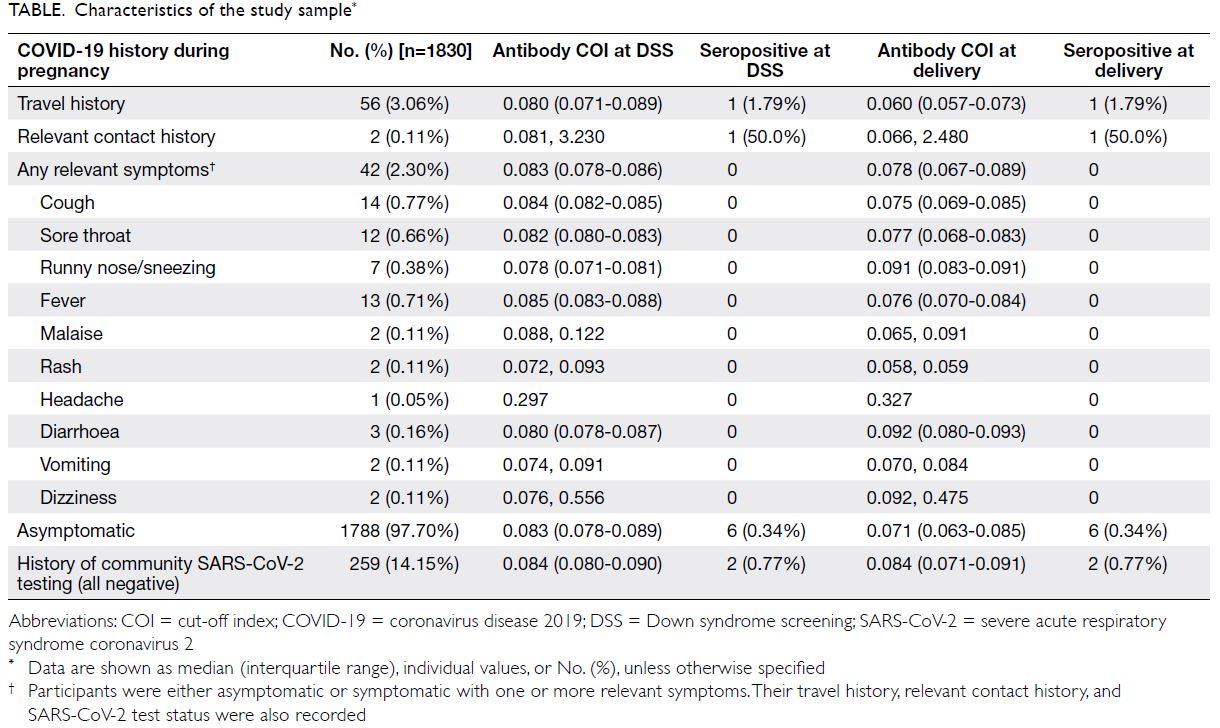
Characteristics of the study sample are
presented in the Table. Fifty six women reported a
travel history during pregnancy; one (1.79%) was
seropositive at both visits. Two women reported
relevant contact history during pregnancy; one (50.0%)
was seropositive at both visits. Forty two women
reported relevant symptoms during pregnancy;
all were seronegative at both visits. Of the 1788
asymptomatic women, six (0.34%) were seropositive
at both visits. In all, 259 women reported undergoing
community SARS-CoV-2 testing during pregnancy;
all tested negative. Among these 259 women,
two (0.77%) were seropositive at both visits.
Discussion
Our findings demonstrated a low seroprevalence
(0.33%) of anti–SARS-CoV-2 antibodies in an
unselected sample of pregnant women in Hong
Kong. Among women with risk factors for
SARS-CoV-2 infection, 1.79% and 50% with a travel
history and relevant contact history, respectively,
were seropositive. This finding suggests that targeted serological testing of pregnant women with a
positive epidemiological link is useful for identifying
women who have recovered from asymptomatic
SARS-CoV-2 infection. A limitation of this study
was that we relied on recruited individuals to recall
COVID-19 symptoms throughout pregnancy,
using only two recall time points: recruitment and
delivery. This aspect may have introduced recall bias,
particularly when COVID-19 symptoms could be
non-specific and overlap with pregnancy symptoms.
However, this limitation presumably did not have a
large effect on the results because the seroprevalence
of anti–SARS-CoV-2 antibody found in our
unselected sample of pregnant women was very low.
With a population of over 7 million, Hong Kong has
largely been successful in controlling the transmission
of SARS-CoV-2. Only 11 981 confirmed or probable
cases have been recorded since the beginning of the
epidemic.5 Thus, it is reasonable that the number of
asymptomatic SARS-CoV-2 infections has also been
low; this low number of asymptomatic infections has
led to a low seroconversion rate in pregnant women.
Compared with other seroprevalence
studies in pregnant women, the seroprevalence
recorded in our study was substantially lower. The
seroprevalence rates were 14% and 21% in Barcelona6
and southern Madrid3 (both in Spain), respectively.
The low seroprevalence of anti–SARS-CoV-2
antibodies recorded in our study is presumably related to the implementation of a series of infection
control strategies, including strict border control,
mandatory quarantine for inbound travellers, mask
wearing, and meticulous contact tracing. Hong
Kong has learnt from its prior experience combating
the SARS (severe acute respiratory syndrome)
outbreak in 2003; accordingly, it implemented
serious control measures early during the current
epidemic. The Figure outlines the timeline of public
health measures implemented during the first three
waves of the epidemic in Hong Kong. By the end of
March 2020, Hong Kong had closed its border to
all incoming non-residents arriving from overseas
and stopped transits through the city. All returning
residents were subject to mandatory quarantine
for 14 days; the quarantine period was extended to
21 days in December 2020. Locally, temporary
closures of gyms, karaoke venues, clubs, and
bars were periodically enforced, depending on
the incidence of COVID-19. Dine-in service was
forbidden from 6:00 pm to 5:00 am for several
months, beginning in mid-July 2020. Mask wearing
in public indoor areas and public transportation was
also mandatory at that time. Notably, all seropositive
cases in this study were first identified at the DSS
visit between February 2020 and July 2020. This
suggests that the seropositive cases acquired
their infections during the first two waves of the
epidemic; the implementation of stricter control measures by the local government during the third
wave might have led to a lower transmission rate of
asymptomatic infection among pregnant women,
resulting in a lack of seroconversion during that
period. Moreover, pregnant women are presumably
more careful about social distancing and compliant
with public health regulations. It would be useful to
compare our seroprevalence results with the findings
in other countries where strict measures were also
implemented, such as Australia and New Zealand.
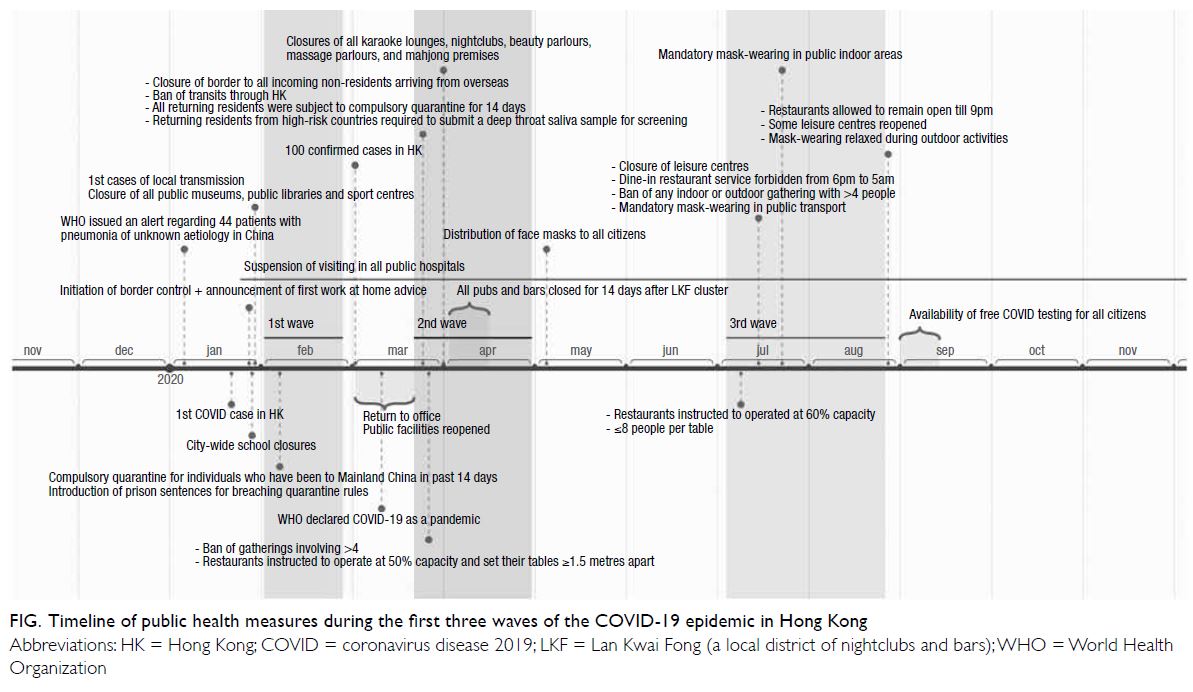
Figure. Timeline of public health measures during the first three waves of the COVID-19 epidemic in Hong Kong
The humoral immune response is characterised
by the production of virus-specific neutralising
antibodies. Regardless of whether patients are
symptomatic, IgG or IgM seroconversion has
been observed in 65% to 100% of patients after
infection with SARS-CoV-2.7 8 9 10 We previously
demonstrated that 75% of pregnant women with
laboratory-confirmed SARS-CoV-2 infection
were seropositive at delivery.11 In addition to the
strict control measures mentioned above, the low
seroprevalence recorded in our study might have
been related to undetectable antibody levels at the
time of specimen collection—the timing of blood
sample collection might not have been compatible
with antibody detection. However, this is unlikely to
have affected the findings among the large number
of pregnant women in this study. A longitudinal
study conducted in Wuhan, China, demonstrated
that the median times from the first virus-positive
test result to IgG or IgM seroconversion were 7 and
14 days in asymptomatic and symptomatic patients,
respectively.12 In the asymptomatic cohort, all
patients underwent seroconversion within 14 days
from the first positive reverse transcriptase–polymerase chain reaction result.12 While waning
immunity was observed at 5 months after infection,13
two studies showed that the antibody levels remained
high and detectable at 8 months after infection.14 15
This finding is consistent with our results that the
antibodies persisted until delivery in all women who
had demonstrated seroconversion at the DSS visit.
Because the two blood samples in this study were
collected approximately 6 months apart, the timing
of specimen collection was presumably adequate to
identify most women who had contracted SARS-CoV-2 and developed detectable levels of antibodies.
Concerns regarding the usefulness of
serological testing have been raised because of the
uncertain onset and duration of humoral immunity;
our study demonstrated a very low seroconversion
yield in an unselected sample of pregnant women.
Because of the ongoing vaccination programme, it
is increasingly difficult to distinguish people who
have acquired humoral immunity through natural
infection from people who have acquired humoral
immunity through vaccination. While the strict public
health measures in Hong Kong have significantly reduced community transmission of SARS-CoV-2,
these measures cannot be maintained indefinitely.
The adverse effects of prolonged social distancing
measures on the economy, education, mental health,
and well-being of the population are immeasurable.
There is increasing evidence that the COVID-19
mRNA vaccines are safe and effective; thus,
pregnant women and women planning to become
pregnant are encouraged to undergo vaccination
at the earliest opportunity because pregnancy
is a risk factor for severe COVID-19–related
complications (eg, intensive care unit admission,
invasive ventilation requirement, and death).16 17 The
risk of preterm birth is also greater among pregnant
women with COVID-19, compared with pregnant
women who do not have COVID-19.18 19 20 Our study
has demonstrated that anti–SARS-CoV-2 antibodies
found at DSS persist until delivery. This result
suggests that the presence of anti–SARS-CoV-2
antibodies in early pregnancy, preferably acquired
through vaccination, would provide some protection
for the pregnant woman and her baby during the
remaining portion of the pregnancy.
Until a highly effective therapeutic drug
targeting SARS-CoV-2 becomes readily available,
mass vaccination remains the best solution to control
the COVID-19 pandemic, avoid further lockdowns,
and allow a return to “normal” pre–COVID-19 life,
as well as saving lives. Our study highlights the
importance of a successful vaccination campaign.
Author contributions
Concept or design: LCY Poon, DS Sahota.
Acquisition of data: HHY Leung, CYT Kwok, WC Leung, DS Sahota, MBW Leung, GCY Lui, SSS Ng.
Analysis or interpretation of data: HHY Leung, CYT Kwok, LCY Poon.
Drafting of the manuscript: HHY Leung, CYT Kwok, LCY Poon, DS Sahota.
Critical revision of the manuscript for important intellectual content: HHY Leung, CYT Kwok, LCY Poon, DS Sahota, SSS Ng, PKS Chan.
Acquisition of data: HHY Leung, CYT Kwok, WC Leung, DS Sahota, MBW Leung, GCY Lui, SSS Ng.
Analysis or interpretation of data: HHY Leung, CYT Kwok, LCY Poon.
Drafting of the manuscript: HHY Leung, CYT Kwok, LCY Poon, DS Sahota.
Critical revision of the manuscript for important intellectual content: HHY Leung, CYT Kwok, LCY Poon, DS Sahota, SSS Ng, PKS Chan.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
LCY Poon has received speaker fees and consultancy payments
from Roche Diagnostics and Ferring Pharmaceuticals. She has
also received in-kind contributions from Roche Diagnostics.
Other authors have disclosed no conflict of interest.
Acknowledgement
We thank Lijia Chen, Tracy CY Ma, Maggie Mak, Ching-man
Mak, Angela ST Tai, Jeffery Ip, Phyllis Ngai, Andrea Chan,
and Lisa LS Chan for making substantial contributions by
involving in study coordination and patient recruitment.
Funding/support
This work was supported by Roche Diagnostic, United States,
which provided reagents for the qualitative detection of anti–
SARS-CoV-2 antibodies.
Ethics approval
Approval for the study was obtained from the Joint Chinese
University of Hong Kong–New Territories East Cluster
Clinical Research Ethics Committee (CREC Ref No. 2020.214).
Patients provided written informed consent to participate in
the study; this included consent for serum storage for research
purposes.
Trial registration
The study is registered with ClinicalTrials.gov (Ref
NCT04465474).
References
1. Byambasuren O, Cardona M, Bell K, Clark J, McLaws ML,
Glasziou P. Estimating the extent of asymptomatic
COVID-19 and its potential for community transmission:
systematic review and meta-analysis. Off J Assoc Med
Microbiol Infect Dis Can 2020;5:223-34. Crossref
2. Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal
morbidity and mortality among pregnant women with
and without COVID-19 infection: the INTERCOVID
multinational cohort study. JAMA Pediatr 2021;175:817-26. Crossref
3. Villala n C, Herraiz I, Luczkowiak J, et al. Seroprevalence analysis of SARS-CoV-2 in pregnant women along the
first pandemic outbreak and perinatal outcome. PLoS One
2020;15:e0243029. Crossref
4. Afshar Y, Gaw SL, Flaherman VJ, el al. Clinical presentation
of coronavirus disease 2019 (COVID-19) in pregnant and
recently pregnant people. Obstet Gynecol 2020;136:1117-25. Crossref
5. Hong Kong SAR Government. Together, we fight the virus!
Available from: https://www.coronavirus.gov.hk/eng/index.html. Accessed 28 Jul 2021.
6. Crovetto F, Crispi F, Llurba E, Figueras F, G mez-Roig MD, Gratac s E. Seroprevalence and presentation of SARS-CoV-2 in pregnancy. Lancet 2020;396:530-1. Crossref
7. W lfel R, Corman VM, Guggemos W, et al. Virological
assessment of hospitalized patients with COVID-2019.
Nature 2020;581:465-9. Crossref
8. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease
2019. Clin Infect Dis 2020;71:2027-34. Crossref
9. Long QX, Liu BZ, Deng HJ, et al. Antibody responses
to SARS-CoV-2 in patients with COVID-19. Nat Med
2020;26:845-8. Crossref
10. Qu J, Wu C, Li X, et al. Profile of immunoglobulin G and
IgM antibodies against severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020;71:2255-8. Crossref
11. Poon LC, Leung BW, Ma T, et al. Relationship between
viral load, infection-to-delivery interval and mother-to-child
transfer of anti-SARS-CoV-2 antibodies. Ultrasound
Obstet Gynecol 2021;57:974-8. Crossref
12. Jiang C, Wang Y, Hu M, et al. Antibody seroconversion
in asymptomatic and symptomatic patients infected
with severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2). Clin Transl Immunology 2020;9:e1182. Crossref
13. Choe PG, Kang CK, Suh HJ, et al. Waning antibody
responses in asymptomatic and symptomatic SARS-CoV-2
infection. Emerg Infect Dis 2021;27:327-9. Crossref
14. Hartley GE, Edwards ES, Aui PM, et al. Rapid generation
of durable B cell memory to SARS-CoV-2 spike and
nucleocapsid proteins in COVID-19 and convalescence.
Sci Immunol 2020;5:eabf8891. Crossref
15. Choe PG, Kim KH, Kang CK, et al. Antibody responses 8 months after asymptomatic or mild SARS-CoV-2 infection.
Emerg Infect Dis 2021;27:928-31. Crossref
16. UK Government. JCVI issues new advice on COVID-19
vaccination for pregnant women. Available from: https://www.gov.uk/government/news/jcvi-issues-new-advice-on-covid-19-vaccination-for-pregnant-women. Accessed
17 May 2021.
17. Hong Kong College of Obstetricians and Gynaecologists.
HKCOG advice on Covid-19 vaccination in pregnant and
lactating women (interim; updated on 21 April 2021).
Available from: https://www.hkcog.org.hk/hkcog/Upload/EditorImage/20210423/20210423141055_6024.pdf.
Accessed 17 May 2021.
18. Martinez-Portilla RJ, Smith ER, He S, et al. Young pregnant
women are also at an increased risk of mortality and
severe illness due to coronavirus disease 2019: analysis of
the Mexican National Surveillance Program. Am J Obstet
Gynecol 2021;224:404-7. Crossref
19. Martinez-Portilla R, Sotiriadis A, Chatzakis C, et al.
Pregnant women with SARS-CoV-2 infection are at higher
risk of death and pneumonia: propensity score matched
analysis of a nationwide prospective cohort (COV19Mx).
Ultrasound Obstet Gynecol 2021;57:224-31. Crossref
20. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations,
risk factors, and maternal and perinatal outcomes of
coronavirus disease 2019 in pregnancy: living systematic
review and meta-analysis. BMJ 2020;370:e3320. Crossref