© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Intravenous iron isomaltoside (Monofer)–induced hypophosphataemia: a case report
KY Wong, MRCP, FHKAM (Medicine); KY Yu, MRCP, FHKAM (Medicine); Maria WH Mak, MRCP, FHKAM (Medicine);
KM Lee, MRCP, FHKAM (Medicine); KF Lee, FRCP, FHKAM (Medicine)
Department of Medicine and Geriatrics, Kwong Wah Hospital, Hong Kong
Corresponding author: Dr KY Wong (wky697@ha.org.hk)
Case report
In January 2019, an 85-year-old woman with a
history of osteoporosis and collapsed L1 had gastric
antral vascular ectasia with multiple failed attempts
of argon photo-coagulation, resulting in severe
iron deficiency anaemia (haemoglobin 4 g/dL).
The patient had been repeatedly admitted for
congestive heart failure precipitated by anaemia that
required blood transfusion. In view of her severe and
ongoing blood loss, intravenous iron isomaltoside
(Monofer) 800 mg monthly was started in February
2019. Before commencement of iron isomaltoside,
iron saturation was 5% (normal 15%-50%). She
was optimally nourished with normal serum
calcium (2.27 mmol/L; normal 2.15-2.55 mmol/L), phosphate (1.4 mmol/L; normal 0.8-1.5 mmol/L),
alkaline phosphatase (108 IU/L; normal 53-141 IU/L)
and vitamin D level (79 nmol/L; normal 50-220 nmol/L).
Hypophosphataemia (0.5 mmol/L) was first noted
in June 2019 (Fig). Owing to the patient’s history
of collapsed vertebra, she was given a first dose of
denosumab in October 2019 by a private orthopaedic
surgeon but serum phosphate further worsened
to 0.1 mmol/L despite aggressive oral phosphate
replacement. She refused hospital admission at
this time. In December 2019, the patient was
hospitalised for anaemia and hypophosphataemia
(0.4 mmol/L) with concomitant serum calcium
2.07 mmol/L and alkaline phosphatase 199 IU/L.
Her estimated glomerular filtration rate was >90 mL/min/1.73 m2. Fractional excretion of
phosphate confirmed renal phosphate wasting
(FePO4 14%; normal <5%) and bicarbonate was
30 mmol/L (normal 22-26 mmol/L). Urinary
protein and glucose were negative. Iron saturation
was 24% and parathyroid hormone 22.6 nmol/L
(normal 1.6-7.2 nmol/L). Fibroblast growth factor
23 (FGF23), measured 3 weeks after the last dose
of iron isomaltoside, was 155 IU/mL (normal
<188 IU/mL). Intravenous iron-induced
hypophosphataemia was suspected. Iron isomaltoside
was stopped and rocaltrol was commenced in
January 2020 with prompt improvement in
phosphate level. Another intravenous iron
preparation, iron sucrose (Venofer), was started
due to her severe anaemia. Attempted re-challenge
with iron isomaltoside resulted in recurrent
hypophosphataemia. Rocaltrol and phosphate
sandoz were gradually tapered down over 6 months.
Serum phosphate remained normal while on iron
sucrose and denosumab.
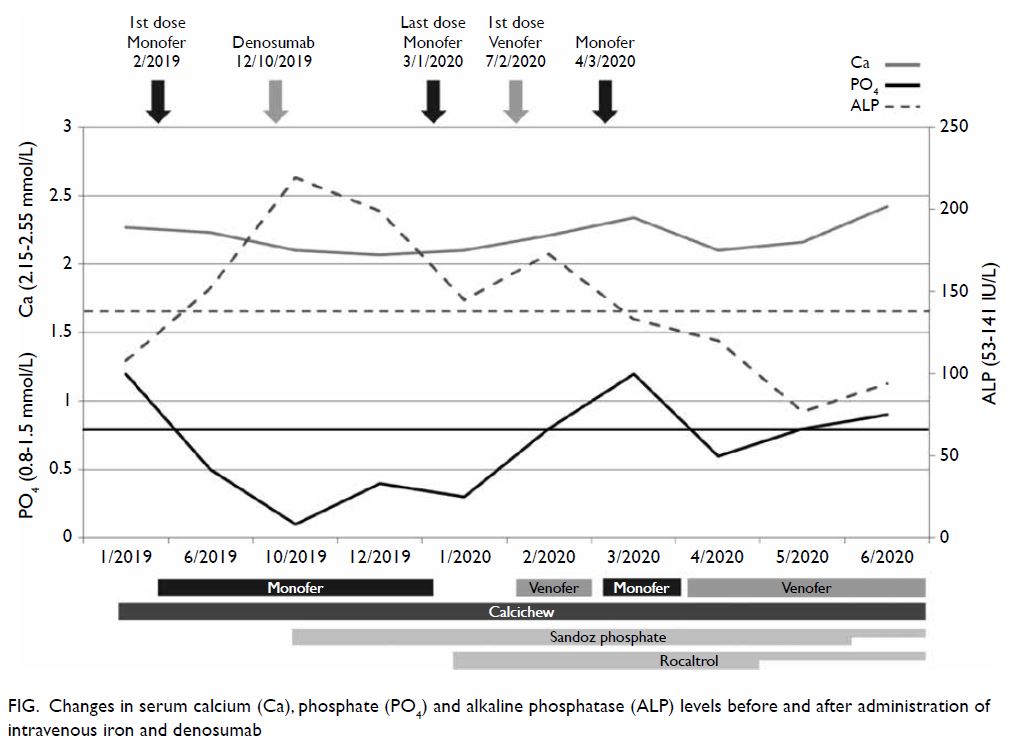
Figure. Changes in serum calcium (Ca), phosphate (PO4) and alkaline phosphatase (ALP) levels before and after administration of intravenous iron and denosumab
Discussion
Iron deficiency anaemia is a commonly encountered problem in daily practice. Although oral iron
remains the recommended route of replacement
due to its low cost and availability, intravenous iron
is considered superior in several respects. First, the
gastrointestinal side-effects of oral iron are avoided.
Second, bioavailability is improved where that of oral
iron is reduced in conditions such as achlorhydria
(eg, proton pump inhibitors, gastric bypass), small
bowel malabsorption (eg, inflammatory bowel
disease, prior small bowel resection, celiac disease)
and chronic inflammation (via upregulation of
hepcidin). Third, intravenous iron allows rapid
repletion of iron, making it a more suitable choice
when there is severe and/or ongoing blood loss.
The new-generation intravenous iron
preparations are all stable iron-carbohydrate
complexes. The three commonly used intravenous
iron preparations locally are iron carboxymaltose
(Ferinject), iron isomaltoside (Monofer) and
iron sucrose (Venofer). They differ in the
attaching carbohydrate ligands that affect the
capacity, stability and immunogenicity of the
complex. Hypophosphataemia is a well-described
complication of iron carboxymaltose but is far
less common in the other two preparations: the
incidence1 of hypophosphataemia is 58%, 4% and
1% for patients with preserved renal function
given iron carboxymaltose, iron isomaltoside
and iron sucrose, respectively. In addition, iron
carboxymaltose–induced hypophosphataemia can
be severe and protracted resulting in osteomalacia
and multiple fractures. Although the pathogenesis2
is not fully understood, it is believed to be mediated through iron carboxymaltose–induced production
of biologically active intact FGF23. The FGF23 is
a phosphaturic hormone produced by osteocytes
and osteoblasts. It reduces phosphate reabsorption
by downregulation of sodium-phosphate co-transporter
in the proximal renal tubule. The
FGF23 also inhibits 1,25-dihydroxyvitamin D
synthesis, leading to vitamin D deficiency and
secondary hyperparathyroidism that contribute to
reduced intestinal phosphate uptake and further
increased renal phosphate wasting, respectively.
Iron isomaltoside also increases intact FGF23
secretion, but to a much lesser extent than iron
carboxymaltose.3 Again, such difference is speculated
to be due to the carbohydrate ligand, since iron
carboxymaltose and iron isomaltoside are equally
effective in replenishing iron store. Although other
indicators of proximal renal tubular dysfunction
such as fractional excretion of urate and urine amino
acid were not measured in this patient, the absence
of glycosuria and non-suppressed FGF23 level were
not typical of a diagnosis of renal Fanconi syndrome.
In addition, improved serum phosphate level after
stopping iron isomaltoside supported the diagnosis
of iron-induced phosphaturia in this patient.
In the meta-analysis by Schaefer et al,4 low
baseline iron saturation and normal renal function
were identified as positive predictors of iron-induced
hypophosphataemia; both were present
in this patient. Severe iron deficiency may cause a
higher increase in FGF23 transcription and renal
impairment is protective due to intrinsic kidney
resistance to FGF23. Furthermore, denosumab may
have worsened the pre-existing hypophosphataemia
induced by iron isomaltoside in this patient since
serum phosphate level fell abruptly in October 2019,
1 week after denosumab injection. Denosumab is an
anti-RANKL (receptor activator of nuclear factor-κB
ligand) antibody that inhibits osteoclastic activity and
hence bone resorption. Severe hypophosphataemia
caused by denosumab has been described in a patient
with tenofovir-induced osteomalacia5 and is possibly
mediated through the following two mechanisms:
first, decreased bone resorption directly reduces
phosphate release; second, fall in bone-derived
calcium worsens secondary hyperparathyroidism
that in turn enhances phosphaturia. These may
explain the profound hypophosphataemia in this
patient after denosumab injection.
There are several reasons for the normal
FGF23 level in this patient. First, the commercial
FGF23 assay detects both intact and cleaved FGF23;
an increased intact FGF23 together with a reduced
cleaved FGF23 may result in a “normal” FGF23 level.
Second, FGF23 may have dropped significantly 3
weeks after the last dose of iron isomaltoside. Third,
FGF23 transcription was reduced with correction
of iron deficiency as reflected by the normal iron saturation.
In addition to phosphate replacement and
switching to a less phosphaturic intravenous iron
preparation, activated vitamin D is needed to
increase phosphate reabsorption during the initial
phase, even in a patient with preserved renal
function. This is because FGF23 inhibits renal
activation of 25-dihydroxyvitamin D to its active
form, 1,25-dihydroxyvitamin D.
In conclusion, although hypophosphataemia is
much less common in patients on iron isomaltoside
than iron carboxymaltose, it is advisable to monitor
phosphate level 1 to 2 weeks after iron isomaltoside
injection in high-risk patients, such as those with
severe anaemia who require repeat dosing, and
patients with pre-existing vitamin D deficiency and/or hyperparathyroidism.
Author contributions
All authors contributed to the concept, acquisition of data, interpretation of data, drafting of the manuscript, and critical
revision for important intellectual content. All authors had
full access to the data, contributed to the study, approved the
final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki. The patient provided informed consent for the treatment/procedures and publication.
References
1. Zoller H, Schaefer B, Glodny B. Iron-induced
hypophosphatemia: an emerging complication. Curr Opin
Nephrol Hypertens 2017;26:266-75. Crossref
2. Edmonston D, Wolf M. FGF23 at the crossroads of phosphate, iron economy and erythropoiesis. Nat Rev
Nephrol 2020;16:7-19. Crossref
3. Wolf M, Rubin J, Achebe M, et al. Effects of iron isomaltoside
vs ferric carboxymaltose on hypophosphatemia in iron-deficiency
anemia: two randomized clinical trials. JAMA
2020;323:432-43. Crossref
4. Schaefer B, Tobiasch M, Viveiros A, et al.
Hypophosphataemia after treatment of iron deficiency with
intravenous ferric carboxymaltose or iron isomaltoside—a
systematic review and meta-analysis. Br J Clin Pharmacol
2021;87:2256-73. Crossref
5. Chung TL, Chen NC, Chen CL. Severe hypophosphatemia
induced by denosumab in a patient with osteomalacia and
tenofovir disoproxil fumarate-related acquired Fanconi
syndrome. Osteoporos Int 2019;30:519-23. Crossref

