Hong Kong Med J 2022 Jun;28(3):257–9 | Epub 27 Apr 2022
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Cold agglutinin–mediated autoimmune
haemolytic anaemia associated with COVID-19
infection: a case report
CY Chang, MRCP (Medicine); HH Chin, MRCP (Medicine)2; PW Chin, MMed (Medicine)2; M Zaid, MMed (Medicine)1
1 Department of Medicine, Hospital Sultanah Aminah, Johor Bahru, Johor, Malaysia
2 Department of Medicine, Hospital Enche’ Besar Hajjah Kalsom, Kluang, Johor, Malaysia
Corresponding author: Dr CY Chang (ccyik28@gmail.com)
Case report
In November 2020, a 70-year-old woman with
diabetes mellitus, hypertension, and dyslipidaemia
presented with a 3-day history of fever, cough, and
rhinorrhoea. She reported no chest pain, shortness
of breath, anosmia, or ageusia. Physical examination
revealed that she was awake and not tachypneic.
There was mild pallor present, but no jaundice. The
patient’s blood pressure was 118/56 mm Hg, pulse
rate 62 beats per minute, and temperature 36.5°C.
Respiratory rate was 16 breaths per minute and
pulse oximetry revealed oxygen saturation of 98%
on ambient air. Chest auscultation revealed bibasilar
crackles. There were no signs of lymphadenopathy,
splenomegaly, or autoimmune disease. Physical
examination was otherwise unremarkable.
Haematological analysis revealed haemoglobin
8.1 g/dL, white cell count 9.6 × 109/L (absolute lymphocyte count 3.1 × 109/L) and platelet count
346 × 109/L. The peripheral blood film showed
moderate anaemia with occasional spherocytes and
marked red blood cell agglutination that dispersed
when blood was heated to 37°C, indicating cold
agglutinin (Fig). The absolute reticulocyte count was
raised at 2.3% and direct antiglobulin test showed
presence of anti-complement (C3d) antibodies but
not anti-immunoglobulin G antibodies. Due to a lack
of facilities at the district hospital, we were unable
to conduct the following tests: serum haptoglobin,
direct antiglobulin test performed with warm-washed
red blood cells, cold agglutinin titre, and
thermal amplitude testing. Mild hyperbilirubinaemia
was present, with indirect bilirubin predominating
(total bilirubin 26.2 mol/L, direct bilirubin 4.7 mol/L,
indirect bilirubin 21.5 mol/L). Liver transaminases
and renal profile were within the normal range. C-reactive protein, serum ferritin, and serum lactate
dehydrogenase level was 5 mg/L, 2671 ?g/L, and
321 U/L, respectively. Mycoplasma serology, blood
cultures, D-dimer, and autoimmune screening were
all negative, as were tests for hepatitis B, hepatitis C,
and human immunodeficiency virus.
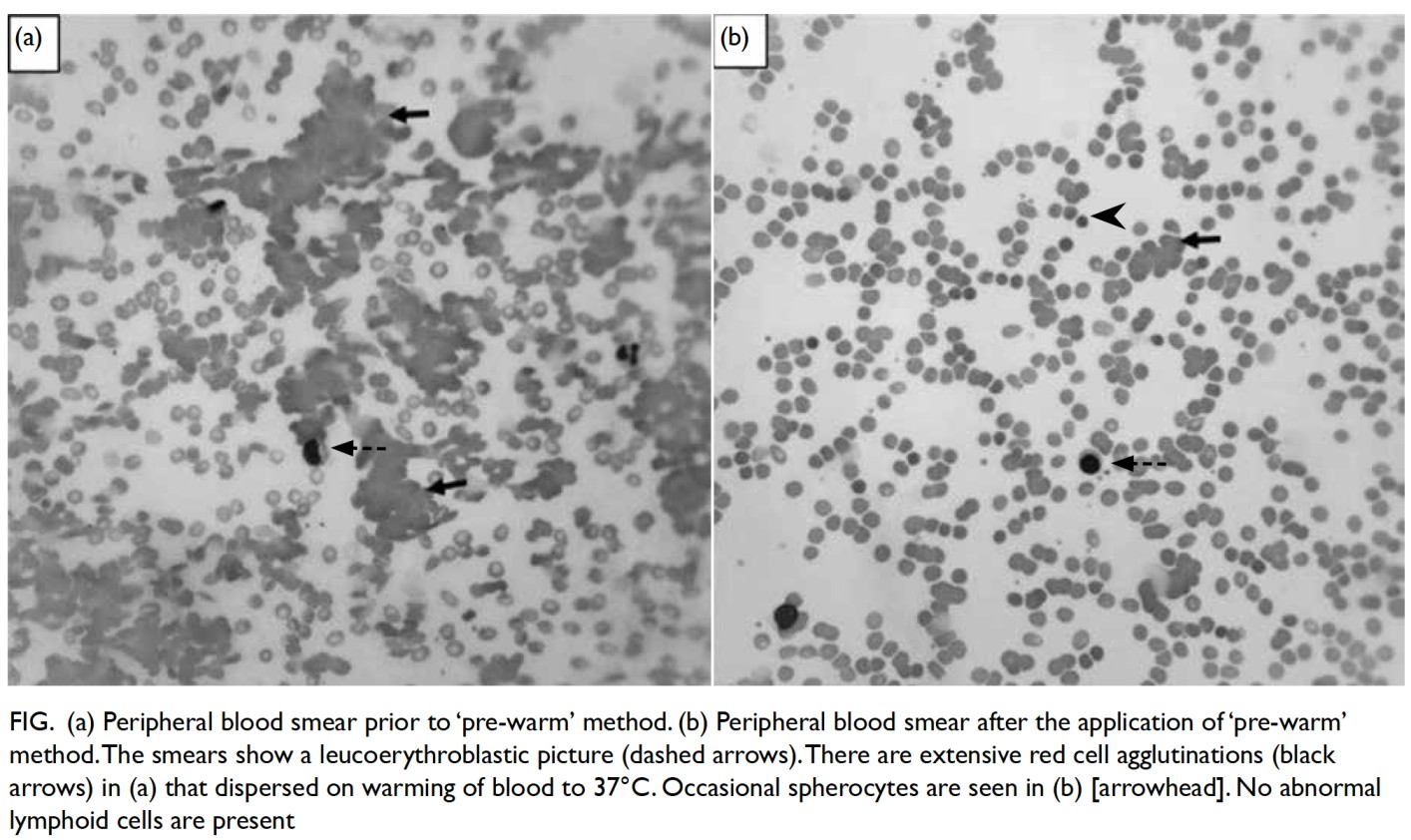
Figure. (a) Peripheral blood smear prior to ‘pre-warm’ method. (b) Peripheral blood smear after the application of ‘pre-warm’ method. The smears show a leucoerythroblastic picture (dashed arrows). There are extensive red cell agglutinations (black arrows) in (a) that dispersed on warming of blood to 37°C. Occasional spherocytes are seen in (b) [arrowhead]. No abnormal lymphoid cells are present
Chest radiograph showed ground-glass
opacities in both lower zones. Coronavirus disease
2019 (COVID-19) infection was confirmed by
reverse transcriptase-polymerase chain reaction
for detection of severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) in nasopharyngeal and
oropharyngeal swab samples (Ct value; E gene 16.09,
RdRp gene 19.23). A diagnosis of cold agglutinin—mediated autoimmune haemolytic anaemia (AIHA)
due to SARS-CoV-2 was made. On the seventh
day of her illness, she developed hypoxaemic
respiratory failure, necessitating 3 L/min
supplemental oxygen administered via nasal
cannula. At the time, inflammatory markers were
elevated, and a new chest radiograph revealed
worsening bilateral airspace opacities. The patient
was prescribed intravenous methylprednisolone
500 mg as a single dose, followed by 2 mg/kg once
daily for the next 5 days. She responded well and
oxygen supplementation was discontinued 7 days
later. Blood inflammatory marker levels (C-reactive
protein 3.1 mg/L) and chest radiograph showed
improved findings. The patient was prescribed
a tapering dose of dexamethasone. One unit of
packed cells was transfused on the third, fifth,
tenth, and fourteenth day of hospitalisation due to
ongoing low-grade haemolysis. In the absence of any
constitutional symptoms, and no lymphadenopathy
or organomegaly on physical examination, a
computed tomography scan was not performed.
She was discharged home on day 21 of her illness
after her symptoms had resolved and she had been
transfusion-independent with stable haemoglobin
level for 1 week. At 1-month follow-up examination,
the patient remained well: haemoglobin was 10 g/L
and new peripheral blood film examination found no
cold agglutinin haemolysis.
Discussion
This pandemic has taken the world by storm,
with many new undocumented symptoms and
treatment strategies. An increasing number
of COVID-19-related complications involving
various disciplines, particularly haematology,
are being reported. Coronavirus disease 2019 is
associated with prominent haematopoietic system
manifestations, including leukopenia, lymphopenia,
thrombocytopenia, disseminated intravascular
coagulation, and prothrombotic state.1 An
association between AIHA and COVID-19 infection
has nonetheless been reported infrequently. The pathophysiology of this association is poorly understood with few cases reported worldwide.
Cold agglutinin disease (CAD) is a form
of AIHA mediated by cold agglutinins that can
agglutinate red blood cells at a temperature of
3°C to 4°C, resulting in complement-mediated
haemolysis. Cold agglutinins arise from either
primary (unknown) or secondary (when cold
agglutinins are produced as a result of an
underlying infection or haematological malignancy)
conditions.2 The pathogenesis of CAD as a result of
infectious agents is unclear. It may be the result of
complement system activation, and associated with
an inflammatory state, including the upregulation of
pro-inflammatory cytokines.
In this case, our patient fulfilled the diagnostic
criteria for CAD that include haemolytic anaemia,
reticulocytosis, elevated lactate dehydrogenase,
hyperbilirubinaemia, positive anti-C3d antibodies,
and negative anti-immunoglobulin G antibodies.3
Other infections and autoimmune diseases
were excluded, and no signs of malignancy were
discovered. We concluded that the CAD in this case
was caused by SARS-CoV-2 (COVID-19). Because
of the ongoing haemolysis, our patient required
packed cell transfusions on multiple occasions.
We believe that her condition deteriorated due
to the “cytokine storm” and complement cascade,
necessitating oxygen supplementation and blood
product transfusion.
Lazarian et al4 reported seven cases of AIHA
(four cases of warm AIHA and three cases of cold
AIHA) associated with COVID-19 infection.
Extensive investigations into the three cases of
cold AIHA revealed the presence of underlying
malignancies (marginal zone lymphoma, 2 cases;
prostate cancer, 1 case). No malignancy was
evident in our patient. Patil et al5 reported a case of
COVID-19 infection with AIHA and pulmonary
embolism, and Maslov et al6 reported a patient with
COVID-19 infection and cold agglutinin haemolytic
anaemia complicated by stroke and bilateral upper
extremity venous thrombosis. Our patient showed
no signs of thromboembolism. Although patients
infected with COVID-19 are at increased risk of
thromboembolic complications, AIHA/CAD should
be considered as a possible contributory factor.
Treatment of CAD is not recommended in
patients who are asymptomatic with mild anaemia
or compensated haemolysis and corticosteroids
should not be used to treat CAD.7 However, in
our patient, the use of methylprednisolone was
indicated as treatment for severe COVID-19
pneumonia. Corticosteroid administration has
been proposed to reduce the systemic inflammatory
response that leads to lung injury and multiorgan
failure in COVID-19. Prompt administration of
methylprednisolone has been shown to significantly reduce mortality rate and ventilator dependence.8
The improvement of haemolysis in our patient
coincided with a favourable treatment response of
COVID-19 to corticosteroid. This was reflected in
her need for fewer packed cell transfusions, as well
as stabilisation of her haemoglobin and no need for
blood transfusions for one week prior to discharge.
Rituximab has also been used to treat COVID-19-associated AIHA in two reported cases following
corticosteroid failure and marginal zone lymphoma,
respectively.4 More research is needed to assess the
safety and efficacy of these therapies in the treatment
of COVID-19-associated AIHA.
Author contributions
Concept or design: CY Chang.
Acquisition of data: CY Chang, HH Chin.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: CY Chang, HH Chin.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: CY Chang, HH Chin.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: CY Chang, HH Chin.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors thank the Director General of Health Malaysia for his permission to publish this article.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patients were treated in accordance with the tenets of the Declaration of Helsinki. The patient(s) provided written
informed consent for all treatments and procedures and for
publication of this case report.
References
1. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al.
Hematological findings and complications of COVID-19.
Am J Hematol 2020;95:834-47. Crossref
2. Berentsen S. New insights in the pathogenesis and therapy of cold agglutinin-mediated autoimmune hemolytic
anemia. Front Immunol 2020;11:590. Crossref
3. Swiecicki PL, Hegerova LT, Gertz MA. Cold agglutinin
disease. Blood 2013;122:1114-21. Crossref
4. Lazarian G, Quinquenel A, Bellal M, et al. Autoimmune
haemolytic anaemia associated with COVID-19 infection.
Br J Haematol 2020;190:29-31. Crossref
5. Patil NR, Herc ES, Girgis M. Cold agglutinin disease and
autoimmune hemolytic anemia with pulmonary embolism
as a presentation of COVID-19 infection. Hematol Oncol
Stem Cell Ther 2020:S1658-3876(20)30116-3. Crossref
6. Maslov DV, Simenson V, Jain S, Badari A. COVID-19 and cold agglutinin hemolytic anomie. TH Open 2020;4:e175-7. Crossref
7. Berentsen S. How I treat cold agglutinin disease. Blood 2021;137:1295-303. Crossref
8. Salton F, Confalonieri P, Meduri GU, et al. Prolonged low-dose
methylprednisolone in patients with severe COVID-19 pneumonia. Open Forum Infect Dis 2020;7:ofaa421. Crossref

