Hong Kong Med J 2022 Jun;28(3):249–56 | Epub 31 May 2022
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Cardiovascular complications of COVID-19
YS Archie Lo, MD (UChicago), FACC1; C Jok, BA2; HF Tse, MD, FRCP3,4
1 Faculty of Medicine School of Public Health and Primary Care, The Chinese University of Hong Kong, Hong Kong
2 St Louis University School of Medicine, United States
3 Cardiology Division, Department of Medicine, School of Clinical Medicine, LKS Faculty of Medicine, The University of Hong Kong, Hong Kong
4 Cardiac and Vascular Center, The University of Hong Kong-Shenzhen Hospital, Shenzhen, PR China
Corresponding author: Dr YS Archie Lo (olasydm@gmail.com)
Abstract
Cardiac injury associated with coronavirus disease
2019 (COVID-19) is associated with high fatality
rates. We reviewed the literature on COVID-19-related cardiovascular complications to elucidate
the putative causes, diagnosis, and management of
cardiovascular complications of COVID-19. Putative
causes of these cardiovascular complications include
cytokine storm, myocarditis, coronary plaque
rupture, hypercoagulability, stress cardiomyopathy
or combinations thereof. Cardiac troponin,
D-dimer, and N-terminal pro B-type natriuretic
peptide levels all provide prognostic information on
COVID-19-related cardiovascular complications:
elevated levels correlate with poorer prognosis.
Coronary thrombosis due to COVID-19 may be
associated with a higher thrombus burden than
that from other causes. Hypercoagulability can be
extremely challenging to treat, and in the absence of
contra-indications, thromboprophylaxis is generally
indicated in intensive care unit patients. With the exception of percutaneous coronary intervention
for acute myocardial infarction, there are no specific
treatments for COVID-19-related cardiovascular
complications and management is primarily
supportive. Whether antiviral therapies, coupled
with monoclonal antibodies administered early in
the course of COVID-19 illness will prevent severe
cardiovascular complications remains to be seen.
Introduction
Coronavirus disease 2019 (COVID-19), caused by
severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a
contagious respiratory illness which can cause serious
complications including stroke, kidney failure, and
cardiovascular complications.1 Cardiovascular
complications are a major risk factor for COVID-19
mortality.2 3 4 The aim of this paper was to review the
literature, published by December 2020, in order to
elucidate the risk factors, putative causes, diagnosis,
and management of cardiovascular complications of
COVID-19.
Incidence, risk factors and mortality in patients with COVID-19
Incidence
Two early studies on COVID-19 reported that
20% to 28% of patients with COVID-19 had
cardiac injury associated with cardiac dysfunction
and arrhythmias.2 3 In a cohort of 416 patients
hospitalised with confirmed COVID-19, cardiac
injury was reported to occur in 19.7%, and was
associated with an unexpectedly high risk of
mortality during hospitalisation. Symptoms of
COVID-19 were more severe when accompanied by
cardiac injury; the mortality rate was higher among patients with cardiac injury than among those without (51.2% vs 4.5%).2
Risk factors: age, sex, and co-morbidities
Independently, in another cohort of 187 patients,
those with cardiac injury were more likely to be male,
older and to have more co-morbidities including
diabetes, hypertension, coronary artery disease,
chronic kidney disease, chronic lung disease, etc.
Severe COVID-19 infections were also potentially
associated with cardiac arrhythmias and the need
for mechanical ventilation. The mortality during
hospitalisation was 7.62% for patients without
underlying cardiovascular disease and normal
cardiac troponin (c-TN) levels, but as high as 69.44%
for those with underlying cardiovascular disease and
elevated c-TN.3
In another report of 72 314 cases (44 672
confirmed) of COVID-19, the crude mortality rate
was 2.3%.4 For octogenarians, the case fatality rate
was 14.8%. A history of coronary artery disease was
present in 4.2% of all cases, but in 22.7% of fatal
cases. Case fatality rates were 10.5% for coronary
artery disease, 7.3% for diabetes, and 6% for
hypertension. Risk of COVID-19 death is highest
among the oldest and lowest among the youngest
populations. Compared with those aged 18 to
29 years, people aged 75 to 84 years and those aged ≥85 years have 200-times and 630-times,
respectively, higher average death rates.5
In a retrospective study of 393 patients, the
prevalence of obesity and male sex also appears to
be higher in patients with COVID-19 who developed
severe symptoms compared with those who did not.6
Putative causes of cardiovascular
complications in patients with COVID-19
Cardiovascular complications of COVID-19
are generally associated with poor prognosis.
Therefore, prevention and treatment of COVID-19
should be considered a priority. To that end,
an understanding of the possible pathogenetic
mechanisms resulting in myocardial injury would
be helpful. Putative causes of cardiovascular
complications in patients with COVID-19 include:
cytokine storm, myocarditis, extreme physical and
emotional stress, ischaemic injury caused by cardiac
microangiopathy or macrovascular coronary artery
disease, hypercoagulopathy, right heart strain, and
cor pulmonale associated with adult respiratory
distress syndrome.
Cytokine storm
In addition to direct viral damage, uncontrolled
inflammation or ‘cytokine storm’—indicated by high
levels of inflammatory markers including C-reactive
protein (CRP), ferritin, and D-dimer, and increased
levels of inflammatory cytokines and chemokines—has been reported in patients with COVID-19.7 8
However, the exact pathogenetic relevance of
cytokine storm has yet to be confirmed.9
Myocarditis
Myocardial inflammation (myocarditis) is evidenced by elevated c-TN level in some patients10 and
autopsy data show mononuclear infiltrate in the
myocardium, with related cardiomyocyte necrosis.11
Although there have been case reports of myocarditis
in patients with COVID-19, it is unclear whether
myocarditis is caused by direct viral invasion or an
uncontrolled inflammatory response.10 12
In a cohort study of 39 autopsy cases of
COVID-19, cardiac infection with COVID-19
was frequently found; however, overt myocarditis
was not observed in the acute phase.13 In contrast,
another study reported on the detection of SARS-CoV-2 genomes
in endomyocardial biopsies.14
A cardiac magnetic resonance (MR) imaging
study of 100 patients recently recovered from
COVID-19 reported cardiac involvement in 78%
of them, with evidence of ongoing myocardial
inflammation in 60% of them. Such involvement
appeared independent of pre-existing conditions,
severity, overall course of the acute illness, and the
time from diagnosis.13 Of 26 competitive athletes,
four (15.4%) had cardiac MR findings suggestive of
myocarditis and eight additional athletes (30.8%)
exhibited late gadolinium enhancement without T2
elevation suggestive of prior myocardial injury.15
In a study of 145 student athletes with
COVID-19 who were either asymptomatic or had
mild to moderate symptoms during acute infection,
cardiac MR findings (at a median of 15 days after a
positive test result for COVID-19) were consistent
with myocarditis in only two patients (1.4%), based
on updated Lake Louise criteria.16
In contrast, preliminary data based on a small
autopsy study of 40 patients showed that cardiac injury
results more from clotting than from inflammation;
microthrombi were frequent, whereas none of the
patients had myocarditis.17 While this observation
has implications for thromboprophylaxis, whether
COVID-19 can cause a viral myocarditis is yet to be
confirmed.
Physical and emotional stress
Cases of typical stress cardiomyopathy have also
been reported,18 suggesting that both physical and
emotional stress may be in part contributory to some
cases of cardiovascular complications of COVID-19.
Ischaemic injury
In some patients, ST-segment elevation myocardial
infarction (STEMI) may be the first clinical
manifestation of COVID-19.19 However, patients
with c-TN elevations may not have epicardial
coronary artery obstruction at angiography. In a case
series of 18 patients with COVID-19 with STEMI,
nine patients underwent coronary angiography; six of them (67%) had obstructive disease. A total of
13 patients died in the hospital (4 due to fatal
myocardial infarction and 9 due to noncoronary
myocardial injury).20 In contrast, patients with
COVID-19 with STEMI had more thrombus burden
and required more anticoagulation than patients
with no COVID-19 infection.21 Very-late stent
thrombosis has also been reported with patients
with COVID-19 and can be one of the presenting
features of COVID-19 in those with a history of
coronary stenting.22
Hypercoagulopathy
Coronavirus disease 2019 is associated with a
hypercoagulable state.23 Although the pathogenesis
is not completely understood, the following may
be observed: elevated fibrinogen and D-dimer;
prolongation of both the prothrombin time and
activated partial thromboplastin time; and mild
thrombocytosis or thrombocytopenia. Major
adverse cardiovascular events, and symptomatic
thromboembolism, occur frequently in patients with
COVID-19, especially among those in the intensive
care unit (ICU), even after thromboprophylaxis.24
Stroke
Unchecked vascular thrombosis may result in
neurological complications. In a case series of
214 patients with COVID-19, neurological
symptoms were seen in 36.4% of patients and were
more common in patients with severe infection.25
A retrospective study of 214 patients reported
six patients with acute stroke, of which five were
ischaemic stroke.26 Stroke has also been reported in
younger patients (aged 33-49 years) with COVID-19.27
Thromboembolism
Post-mortem studies of 12 patients have reported
pulmonary embolism as the direct cause of death
in four patients (33%) and deep venous thrombosis
in seven patients (58%).28 The risk for venous
thromboembolism is markedly elevated with prevalence up to 32%,24 29 30 highest with patients in the ICU.30 In a large study involving
3334 consecutive hospitalised patients with
COVID-19, among 829 patients in the ICU, 29.4%
had a thrombotic event (13.6% venous and 18.6%
arterial).30 Although low-dose anticoagulation has
been used for thromboprophylaxis, in a series of 184
critically ill patients with COVID-19, 31% suffered
clinically significant thrombotic complications
despite low-dose nadroparin.31
Thrombocytopenia
A meta-analysis demonstrated thrombocytopenia in patients with severe disease is associated with
increased risk of COVID-19 mortality.32 How
thrombocytopenia should be factored into the
decision to prescribe anticoagulant therapy has yet
to be studied.
Cor pulmonale, right heart strain, pulmonary
hypertension
An echocardiographic study of 110 COVID-19 cases
noted right ventricular dilation in 31% of patients.33
Another study demonstrated that when compared
with those in the lowest quartile, patients with the
highest right ventricular longitudinal strain quartile
had an increased risk of elevated D-dimer and
CRP levels, acute cardiac injury, acute respiratory
distress syndrome, deep vein thrombosis as well as
mortality.34 Acute cor pulmonale, right heart strain,
and/or pulmonary hypertension should always be
considered in critically ill patients with COVID-19.35
Other significant cardiac issues in
COVID-19
Arrhythmias
Early data suggested an incidence of 16.7%
arrhythmias among hospitalised patients with
COVID-19 and 44.4% of ICU admissions.36 A
multicentre study of 192 patients with COVID-19
reported a prevalence of 12.5% for atrial fibrillation
among hospitalised patients with COVID-19.37
Another study evaluating 115 patients with
COVID-19 reported atrial tachyarrhythmia in 16.5%
of patients, with atrial fibrillation being the most
common (63%).38 Those with atrial tachyarrhythmia
had higher CRP and D-dimer levels compared
with those without atrial tachyarrhythmia. Among
393 patients with COVID-19, atrial arrhythmias
were more common among patients on ventilators
(18.5% vs 1.9%).6 In another study of 700 patients
with COVID-19, nine patients experienced cardiac
arrest. All cardiac arrests occurred in patients
in the ICU. No patients experienced sustained
monomorphic ventricular tachycardia, ventricular
fibrillation, or complete heart block. Twenty-five
patients had atrial fibrillation, nine had significant
bradyarrhythmia, and 10 had non-sustained
ventricular tachycardia.39 Among 187 patients with
COVID-19, when compared with patients with
normal c-TN levels, those with elevated c-TN levels
developed more frequent malignant arrhythmias
(17.3% vs 1.5%), including ventricular tachycardia/ventricular fibrillation.3
Heart failure
Patients with cardiovascular disease and heart failure
are more susceptible to COVID-19 and have a more
severe clinical course once infected.40 41 In two studies
of patients with COVID-19 hospitalised in Wuhan, heart failure was identified as a complication in
about 50% of the fatalities.42 In a retrospective
multicentred study, among 8383 patients with heart
failure who were hospitalised with COVID-19,
nearly one in four died during hospitalisation.43
Evidently, heart failure in patients with COVID-19
may be triggered or aggravated by the acute infection
in patients with pre-existing cardiovascular disease
or incident acute myocardial insult.
Cardiac arrest
Malignant tachyarrhythmias resulting in cardiac
arrest present a dilemma for caregivers. The
outcomes of out-of-hospital cardiac arrest were
worse during the first weeks of the COVID-19
pandemic in the United States, and this was observed
not only in areas with high case-fatality rates but
also ones with lower rates.44 In a retrospective
study of 136 patients with COVID-19, 119 (87.5%)
had a respiratory cause for their cardiac arrest, and
the initial rhythm was asystole in 89.7%, pulseless
electrical activity in 4.4%, and shockable in 5.9%. The
return-of-spontaneous-circulation rate was 13.2%
and 30-day survival rate was only 2.9%.45 In another
study of 54 patients with COVID-19, the mortality
rate following cardiopulmonary resuscitation was
even worse (100%). The initial rhythm was non-shockable
for 52 patients (96.3%), with pulseless
electrical activity being the most common (81.5%).
Although the return-of-spontaneous-circulation
rate was achieved in 29 patients (53.7%), none
survived to be discharged home.46
Prognostic laboratory parameters
for cardiovascular complications
in patients with COVID-19
Prognostic parameters for cardiovascular
complications in patients with COVID-19 include
c-TN level, D-dimer level, and N-terminal pro
B-type natriuretic peptide (NT-proBNP) level.
Cardiac troponin level
Increases in c-TN level indicative of myocardial
injury is common in patients with COVID-19
and is associated with adverse outcomes such as
arrhythmias and death. The risk of cardiac injury,
as diagnosed by increased c-TN levels (>99th
percentile), was found in up to 22% of patients in the
ICU, and in 59% of those that died.36 In another study
of 2736 patients with COVID-19, c-TN elevation was
observed in 36%, and c-TN elevation (>0.09 ng/dL)
appears to triple the mortality risk.47 Other studies
of patients with COVID-19 have also demonstrated
a poorer prognosis, including mortality, in patients
with c-TN elevation.41 48 Both c-TN and NT-proBNP
levels were documented to be elevated significantly
during the course of hospitalisation among those who eventually died, but no dynamic changes were
observed among the survivors.3 Moreover, patients
with COVID-19 with myocardial injury who also
have transthoracic echocardiography abnormalities
had a higher mortality risk.49
D-dimer level
Elevated D-dimer levels were higher among patients
with COVID-19 and was correlated with a poorer
prognosis. Multivariate analysis showed increasing
odds of in-hospital death associated with D-dimer
value above 1 μg/mL.50 In a study of 343 patients
with COVID-19, D-dimer levels ≥2.0 μg/mL had a
higher incidence of mortality compared with those
with D-dimer levels <2.0 μg/mL (12/67 vs 1/267,
P<0.001).51 A markedly elevated D-dimer (>6 times
the upper limit of normal) is a consistent predictor
of thrombotic events and poor overall prognosis.52
Indeed, the International Society on Thrombosis
and Haemostasis has advised that for patients who
have markedly raised D-dimers (arbitrarily defined
as three- to four-fold increase), admission to hospital
should be considered even in the absence of other
severe symptoms.53 The importance of D-dimer is
emphasised in several other international guidelines.52 53 54 55
N-terminal pro B-type natriuretic peptide
level
As a biomarker of heart failure, NT-proBNP levels
are commonly elevated in hospitalised patients with
COVID-19, particularly in those with elevated c-TN
levels. The report by Shi et al2 showed that NT-proBNP
levels were significantly higher in patients
with elevated c-TN levels than in those without
c-TN elevation (1689 vs 139 pg/mL). A study of 3219
hospitalised patients with COVID-19, elevated c-TN
was detected in 6.5%, and an elevated NT-proBNP
level in 12.9%.56 The adjusted hazard ratio for 28-day
mortality for c-TN was 7.12 and for NT-proBNP
5.11, confirming that elevated NT-proBNP levels also
carry prognostic information. Although NT-proBNP
provides corroborating laboratory information on
heart failure, the caveat is that NT-proBNP levels
increase with age and with various other conditions
including renal failure, thus compromising its utility
in older patients with confounding variables.
Management of cardiovascular
complications of COVID-19
Coronary thrombosis
The approach to the diagnosis and management
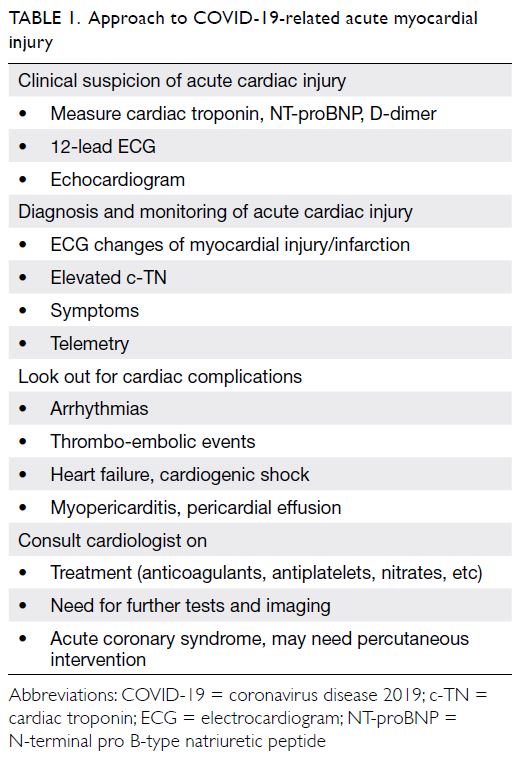
of STEMI in patients with COVID-19 is similar to that for patients without (Table 1). The approaches
endorsed by the American College of Cardiology are
recommended57: their emphasis is on patient selection for the cardiac catheterisation laboratory, resource allocation, and protection of the interventional team and other healthcare workers involved in caring for the COVID-19 patient.
On occasion, it is reasonable to liberalise the
use of intravenous thrombolytic therapy relative
to primary percutaneous coronary intervention.
Intravenous thrombolytic therapy can be considered
for a relatively stable patient with STEMI and
COVID-19. Obviously, in those STEMI patients
who are critically ill with COVID-19, the decision
to reperfuse with either primary percutaneous
coronary intervention or intravenous thrombolytic
therapy should be individualised, and contingent
upon hospital resources. In this regard, the consensus
statement from the Taiwan Society of Cardiology is
both pragmatic and reasonable.58
In the event that primary percutaneous
coronary intervention is to be performed,
maximum personal protective equipment is
essential. Intubation, suction, and cardiopulmonary
resuscitation all result in aerosolisation of respiratory secretions and increase the risks to the hospital staff.
Patients already intubated pose less of an infectious
risk. Hence patients with COVID-19 or suspected
COVID-19 requiring intubation should be intubated
prior to arrival to the catheterisation suite.
In the treatment of STEMI patients, an early Hong Kong study reported that both the “symptom
onset to first medical contact” and the “door-to-device”
times pertaining to primary percutaneous
coronary intervention were reported to be
substantially prolonged.59
Studies from both England60 and the United
States61 have confirmed that hospital admissions for
acute coronary syndrome declined by 40% to 48% in
the early days of COVID-19. It is likely that patients
with acute coronary syndromes avoided attending
hospital during this period.
Heart failure
Standard indications for use of various agents for treatment of heart failure apply to patients with
COVID-19. The coexistence of heart failure and
COVID-19 complicates diagnosis and management
because of overlapping chest findings; however,
there are notable differences in chest computed
tomography between heart failure and COVID-19
pneumonia, such as lesion distribution/morphology,
and pulmonary vein engorgement, which can all
help to differentiate between the two.62
Cardiopulmonary resuscitation
Cardiopulmonary resuscitation poses
Cardiopulmonary resuscitation poses a very high
risk for viral spread, and full personal protective
equipment should be provided. Immediate
intubation should be prioritised in order to
minimise the duration of any aerosolisation. While
awaiting intubation, bag/mask ventilation with filter
is advised.
Hypercoagulopathy
Several international guidelines have issued
recommendations advocating chemoprophylaxis
in all hospitalised patients with COVID-19,63 64 65 66
in the absence of both contra-indications and
bleeding complications (Table 2). In the event
thromboprophylaxis is deemed indicated, low-molecular-weight heparin is preferred, but
unfractionated heparin can be used if low-molecular-weight
heparin is unavailable or if kidney function is
severely impaired. Low-molecular-weight heparin
may be preferred over unfractionated heparin for
staff safety reasons.
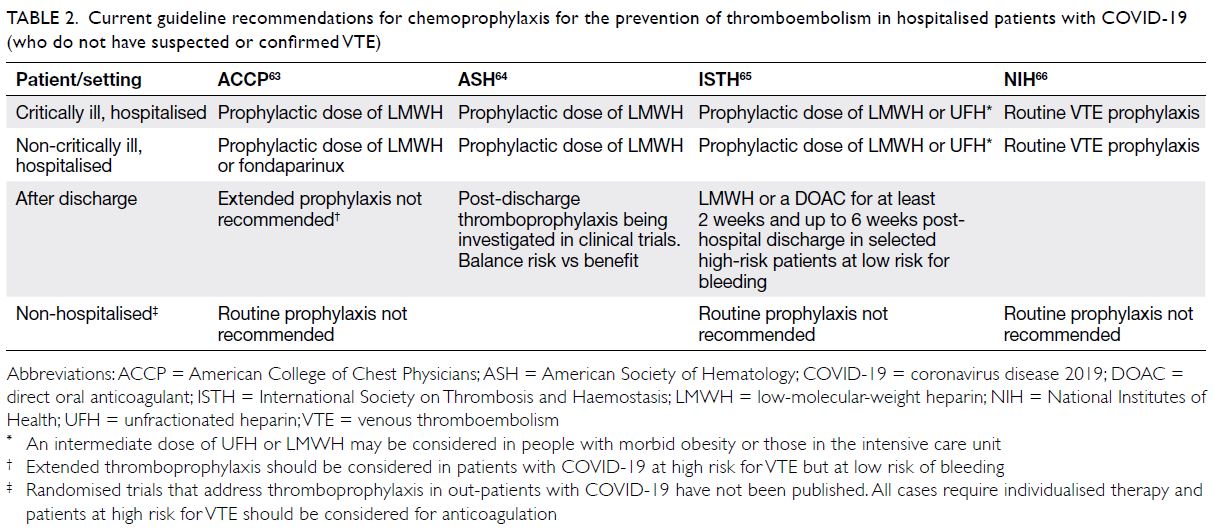
Table 2. Current guideline recommendations for chemoprophylaxis for the prevention of thromboembolism in hospitalised patients with COVID-19 (who do not have suspected or confirmed VTE)
Athletes recovering from COVID-19
As for athletes who have recovered from COVID-19
infections, a recent expert consensus article
recommended 2-week convalescence followed
by no diagnostic cardiac testing if asymptomatic,
and an electrocardiogram and transthoracic
echocardiogram in mildly symptomatic athletes with
COVID-19 to return to participate in competitive
sports.67
Summary
Cardiovascular complications of COVID-19 are
associated with higher fatality rates. Putative
causes of cardiac injury include cytokine storm,
myocarditis, extreme physical and emotional stress,
ischaemic injury, hypercoagulopathy, right heart
strain, and cor pulmonale, or combinations thereof.
Echocardiography and c-TN, D-dimer, and NT-proBNP
levels all provide prognostic information.
Aside from percutaneous coronary intervention for
STEMI, there is no specific treatment for COVID-19-associated cardiac injury, and management is
primarily supportive. Whether antiviral therapies
administered early in the course of disease
will prevent severe disease and cardiovascular
complications associated with COVID-19 remain to
be seen.
Author contributions
Concept or design: YSA Lo.
Acquisition of data: YSA Lo, C Jok.
Analysis or interpretation of data: YSA Lo.
Drafting of the manuscript: YSA Lo.
Critical revision of the manuscript for important intellectual content: YSA Lo, HF Tse.
Acquisition of data: YSA Lo, C Jok.
Analysis or interpretation of data: YSA Lo.
Drafting of the manuscript: YSA Lo.
Critical revision of the manuscript for important intellectual content: YSA Lo, HF Tse.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
1. Drake TM, Riad AM, Fairfield CJ, et al. Characterisation
of in-hospital complications associated with COVID-19
using the ISARIC WHO Clinical Characterisation Protocol
UK: a prospective, multicentre cohort study. Lancet
2021;398:223-7. Crossref
2. Shi S, Qin M, Shen B, et al. Association of cardiac injury
with mortality in hospitalized patients with COVID-19 in
Wuhan, China. JAMA Cardiol 2020;5:802-10. Crossref
3. Guo T, Fan Y, Chen M, et al. Cardiovascular implications
of fatal outcomes of patients with coronavirus disease 2019
(COVID-19). JAMA Cardiol 2020;5:811-8. Crossref
4. Wu Z, McGoogan JM. Characteristics of and important
lessons from the coronavirus disease 2019 (COVID-19)
outbreak in China: summary of a report of 72 314 cases
from the Chinese Center for Disease Control and
Prevention. JAMA 2020;323:1239-42. Crossref
5. Centers for Disease Control and Prevention, US
Department of Health & Human Services. COVID-19
hospitalization and death by age. 11 February 2020 (updated
18 August 2020). Available from: https://www.cdc.
gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html. Accessed 2
Dec 2020.
6. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York city. N Engl J Med 2020;382:2372-4. Crossref
7. Mehta P, McAuley DF, Brown M, et al. COVID-19: consider
cytokine storm syndromes and immunosuppression.
Lancet 2020;395:1033-4. Crossref
8. Zhang X, Tan Y, Ling Y, et al. Viral and host factors
related to the clinical outcome of COVID-19. Nature
2020;583;437-40. Crossref
9. Sinha P, Matthay MA, Calfee CS. Is a “Cytokine Storm”
relevant to COVID-19? JAMA Intern Med 2020;180:1152-4. Crossref
10. Zeng J, Liu Y, Yuan J, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and
insights. Infection 2020;48:773-7. Crossref
11. Yao XH, Li TY, He ZC, et al. A pathological report of
three COVID-19 cases by minimally invasive autopsies [in
Chinese]. Zhonghua Bing Li Xue Za Zhi 2020;49:411-7.
12. Lindner D, Fitzek A, Bräuninger H, et al. Association of
cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol 2020;5:1281-5. Crossref
13. Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of
cardiovascular magnetic resonance imaging in patients
recently recovered from coronavirus disease 2019
(COVID-19). JAMA Cardiol 2020;5:1265-73. Crossref
14. Escher F, Pietsch H, Aleshcheva G, et al. Detection of viral
SARS-CoV-2 genomes and histopathological changes in
endomyocardial biopsies. ESC Heart Fail 2020;7:2440-7. Crossref
15. Rajpal S, Tong MS; Borchers J, et al. Cardiovascular
magnetic resonance findings in competitive athletes
recovering from COVID-19 Infection. JAMA Cardiol
2021;6:116-8. Crossref
16. Starekova J, Bluemke DA, Bradham WS, et al. Evaluation
for myocarditis in competitive student athletes recovering
from coronavirus disease 2019 with cardiac magnetic
resonance imaging. JAMA Cardiol 2021;6:945-50. Crossref
17. Phend C. COVID heart autopsies point more to clot damage than myocarditis. Available from: https://www.medpagetoday.com/meetingcoverage/tct/89143. Accessed 7 Dec 2020.
18. Jabri A, Kalra A, Kumar A, et al. Incidence of stress
cardiomyopathy during the coronavirus disease 2019
pandemic. JAMA Netw Open 2020;3:e2014780. Crossref
19. Stefanini GG, Montorfano M, Trabattoni D, et al.
ST-elevation myocardial infarction in patients with
COVID-19: clinical and angiographic outcomes.
Circulation 2020;141:2113-6. Crossref
20. Bangalore S, Sharma A, Slotwiner A, et al. ST-segment
elevation in patients with Covid-19—a case series. N Engl J
Med 2020;382:2478-80. Crossref
21. Choudry FA, Hamshere SM, Rathod KS, et al. High
thrombus burden in patients with COVID-19 presenting
with ST-segment elevation myocardial infarction. J Am
Coll Cardiol 2020;76:1168-76. Crossref
22. Prieto-Lobato A, Ramos-Martínez R, Vallejo-Calcerrada N,
Corbí-Pascual M, Córdoba-Soriano JG. A case series of
stent thrombosis during the COVID-19 pandemic. JACC
Case Rep 2020;2:1291-6. Crossref
23. Maier CL, Truong AD, Auld SC, Polly DM, Tanksley CL,
Duncan A. COVID-19-associated hyperviscosity: a
link between inflammation and thrombophilia? Lancet
2020;395:1758-9. Crossref
24. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of
venous thromboembolism in patients with severe novel
coronavirus pneumonia. J Thromb Haemost 2020;18:1421-4. Crossref
25. Mao L, Jin H, Wang M, et al. Neurologic manifestations
of hospitalized patients with coronavirus disease 2019 in
Wuhan, China. JAMA Neurol 2020;77:683-90. Crossref
26. Li Y, Li M, Wang M, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol 2020;5:279-84. Crossref
27. Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a
presenting feature of Covid-19 in the young. N Engl J Med
2020;382:e60. Crossref
28. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy
findings and venous thromboembolism in patients with
COVID-19: a prospective cohort study. Ann Intern Med
2020;173:268-77. Crossref
29. Artifoni M, Danic G, Gautier G, et al. Systematic
assessment of venous thromboembolism in COVID-19
patients receiving thromboprophylaxis: incidence and role
of D-dimer as predictive factors. J Thromb Thrombolysis
2020;50:211-6. Crossref
30. Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E,
Hochman J, Berger JS. Thrombosis in hospitalized patients
with COVID-19 in a New York City Health System. JAMA
2020;324:799-801. Crossref
31. Klok FA, Kruip MJ, van der Meer NJ, et al. Incidence of
thrombotic complications in critically ill ICU patients with
COVID-19. Thromb Res 2020;191:153-5. Crossref
32. Lippi G, Plebani M, Henry BM. Thrombocytopenia
is associated with severe coronavirus disease 2019
(COVID-19) infections: a meta-analysis. Clin Chim Acta
2020;506:145-8. Crossref
33. Argulian E, Sud K, Vogel B, et al. Right ventricular dilation
in hospitalized patients with COVID-19 infection. JACC
Cardiovasc Imaging 2020;13:2459-61. Crossref
34. Li Y, Li H, Zhu S, et al. Prognostic value of right ventricular
longitudinal strain in patients with COVID-19. JACC
Cardiovasc Imaging 2020;13:2287-99. Crossref
35. Creel-Bulos C, Hockstein M, Amin N, Melhem S, Truong A,
Sharifpour M. Acute cor pulmonale in critically Ill patients
with Covid-19. N Engl J Med 2020;382:e70. Crossref
36. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138
hospitalized patients with 2019 novel coronavirus–infected
pneumonia in Wuhan, China. JAMA 2020;323:1061-9. Crossref
37. Russo M, Di Maio M, Attena E, et al. Clinical impact of
pre-admission antithrombotic therapy in hospitalized
patients with COVID-19: a multicenter observational
study. Pharmacol Res 2020;159:104965. Crossref
38. Colon CM, Barrios JG, Chiles JW, et al. Atrial arrhythmias
in COVID-19 patients. JACC Clin Electrophysiol
2020;6:1189-90. Crossref
39. Bhatla A, Mayer MM, Adusumalli S, et al. COVID-19 and
cardiac arrhythmias. Heart Rhythm 2020;17:1439-44. Crossref
40. Tomasoni D, Italia L, Adamo M, et al. COVID-19 and heart
failure: from infection to inflammation and angiotensin II
stimulation. Searching for evidence from a new disease.
Eur J Heart Fail 2020;22:957-66. Crossref
41. Du RH, Liang LR, Yang CQ, et al. Predictors of mortality
for patients with COVID-19 pneumonia caused by
SARS-CoV-2: a prospective cohort study. Eur Respir J
2020;55:2000524. Crossref
42. Chen T, Wu D, Chen H, et al. Clinical characteristics of
113 deceased patients with coronavirus disease 2019:
retrospective study. BMJ 2020;368:m1091. Crossref
43. Bhatt AS, Jering KS, Vaduganathan M, et al. Clinical
outcomes in patients with heart failure hospitalized with
COVID-19. JACC Heart Fail 2021;9:65-73. Crossref
44. Chan PS, Girotra S, Tang Y, et al. Outcomes for out-of-hospital cardiac arrest in the United States during
the coronavirus disease 2019 pandemic. JAMA Cardiol 2021;6:296-303. Crossref
45. Shao F, Xu S, Ma X, et al. In-hospital cardiac arrest
outcomes among patients with COVID-19 pneumonia in
Wuhan, China. Resuscitation 2020;151:18-23. Crossref
46. Thapa SB, Kakar TS, Mayer C, Khanal D. Clinical outcomes
of in-hospital cardiac arrest in COVID-19. JAMA Intern
Med 2021;181:279-81. Crossref
47. Lala A, Johnson KW, Januzzi JL, et al. Prevalence and
impact of myocardial injury in patients hospitalized with
COVID-19 infection. J Am Coll Cardiol 2020;76:533-46. Crossref
48. Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I
in patients with coronavirus disease 2019 (COVID-19):
evidence from a meta-analysis. Prog Cardiovasc Dis
2020;63:390-1. Crossref
49. Giustino G, Croft LB, Stefanini GG, et al. Characterization
of myocardial injury in patients with COVID-19. J Am Coll
Cardiol 2020;76:2043-55. Crossref
50. Zhou F, Yu T, Du R, et al. Clinical course and risk factors
for mortality of adult inpatients with COVID-19 in Wuhan,
China: a retrospective cohort study. Lancet 2020;395:1054-62. Crossref
51. Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission
to predict in-hospital mortality in patients with Covid-19. J
Thromb Haemost 2020;18:1324-9. Crossref
52. Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and
Standardization Committee communication: clinical
guidance on the diagnosis, prevention and treatment of
venous thromboembolism in hospitalized patients with
COVID-19. J Thromb Haemost 2020;18:1859-65. Crossref
53. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on
recognition and management of coagulopathy in COVID-
19. J Thromb Haemost 2020;18:1023-6. Crossref
54. American Society of Hematology. COVID-19 and VTE/
anticoagulation: frequently asked questions. Available
from: https://www.hematology.org/covid-19/covid-19-and-vte-anticoagulation. Accessed 23 Jun 2020.
55. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and
thrombotic or thromboembolic disease: implications for
prevention, antithrombotic therapy, and follow-up: JACC
state-of-the-art review. J Am Coll Cardiol 2020;75:2950-73. Crossref
56. Qin JJ, Cheng X, Zhou F, et al. Redefining cardiac
biomarkers in predicting mortality of inpatients with COVID-19. Hypertension 2020;76:1104-12. Crossref
57. Mahmud E, Dauerman HL, Welt FG, et al. Management of acute myocardial infarction during the COVID-19 pandemic: a position statement from the Society for Cardiovascular Angiography and Interventions (SCAI), the American College of Cardiology (ACC), and the American College of Emergency Physicians (ACEP). J Am Coll Cardiol 2020;76:1375-84. Crossref
58. Li YH, Wang MT, Huang WC, Hwang JJ. Management
of acute coronary syndrome in patients with suspected
or confirmed coronavirus disease 2019: Consensus from
Taiwan Society of Cardiology. J Formos Med Assoc
2021;120:78-82. Crossref
59. Tam CC, Cheung KS, Lam S, et al. Impact of coronavirus
disease 2019 (COVID-19) outbreak on ST-segment–elevation myocardial infarction care in Hong Kong, China.
Circ Cardiovasc Qual Outcomes 2020;13:e006631. Crossref
60. Mafham MM, Spata E, Goldacre R, et al. COVID-19
pandemic and admission rates for and management of acute
coronary syndromes in England. Lancet 2020;396:381-9. Crossref
61. Solomon MD, McNulty EJ, Rana JS, et al. The covid-19
pandemic and the incidence of acute myocardial infarction.
N Engl J Med 2020;383:691-3. Crossref
62. Zhu ZW, Tang JJ, Chai XP, et al. Comparison of heart
failure and COVID-19 in chest CT features and clinical
characteristics. Zhonghua Xin Xue Guan Bing Za Zhi
2020;48:467-71.
63. Moores LK, Tritschler T, Brosnahan S, et al. Prevention,
diagnosis, and treatment of VTE in patients with
coronavirus disease 2019 CHEST guideline and expert
panel report. Chest 2020;158:1143-63. Crossref
64. American Society of Hematology. ASH Guidelines on Use
of Anticoagulation in Patients with COVID-19. Available
from: https://www.hematology.org/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines/ash-guidelines-on-use-of-anticoagulation-in-patients-with-covid-19. Accessed 7 Dec 2020.
65. Thachil J, Juffermans NP, Ranucci M, et al. ISTH DIC
subcommittee communication on anticoagulation in
COVID-19. J Thromb Haemost 2020;18:2138-44. Crossref
66. National Institutes of Health. Antithrombotic therapy
in patients with COVID-19. Last updated: 12 May 2020.
Available from: https://www.covid19treatmentguidelines.
nih.gov/adjunctive-therapy/antithrombotic-therapy/.
Accessed 7 Dec 2020.
67. Phelan D, Kim JH, Chung EH. A game plan for the
resumption of sport and exercise after coronavirus disease
2019 (COVID-19) infection. JAMA Cardiol 2020;5:1085-6. Crossref