Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Fulminant necrotising amoebic colitis: a report
of two cases
LM Tam, MB, BS1; KC Ng, MB, BS, FRCS1; CH Man, MB, BS FRCS1; FY Cheng, MB, BS2; Y Gao, LMCHK2
1 Department of Surgery, Caritas Medical Centre, Hong Kong
2 Department of Pathology, Caritas Medical Centre, Hong Kong
Corresponding author: Dr LM Tam (tammy520@connect.hku.hk)
Case report
Case 1
In January 2019, a 31-year-old man with a history
of amphetamine abuse presented with a 10-day
history of watery diarrhoea and abdominal pain. On
admission, he had a fever of 38.1°C and tachycardia
but normal blood pressure. Abdominal examination
revealed tenderness and guarding over the lower
abdomen. The patient was resuscitated and
intravenous antibiotics prescribed since an infective
cause was considered most likely. Abdominal
plain radiograph showed grossly dilated small and
especially large bowel (diameter up to 9 cm). White
cell count was 22×109/L (neutrophil differential
count 19×109/L), liver and renal function were
unremarkable. Contrast computed tomography (CT)
scan of the abdomen and pelvis revealed extensive
colitis, suggesting pseudomembranous colitis or
diffuse colitis. Stool culture for Clostridium difficile
was negative and stool microscopy revealed no ova
or cysts. Human immunodeficiency virus (HIV),
hepatitis B and C virus serologies were non-reactive.
He was treated as pseudomembranous colitis by
the medical gastrointestinal team and commenced
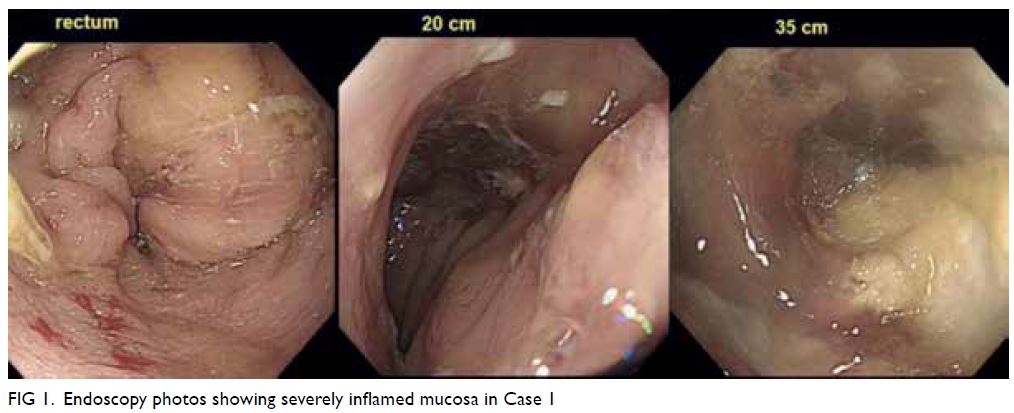
on oral vancomycin. Later sigmoidoscopy revealed
inflamed mucosa with multiple ulcers, especially at
the sigmoid colon (Fig 1). Biopsies were taken. The
patient’s condition deteriorated and he developed
septic shock. A new contrast CT scan showed
pneumoperitoneum. Emergency laparotomy was performed and revealed generalised faecal peritonitis
with the whole colon necrosed and communicating
with the peritoneal cavity. Debridement of necrotic
tissue and multiple segmental resections of bowel
with end ileostomy were performed in stages.
The diagnosis of fulminant amoebic colitis (FAC)
was made on histopathological evaluation of the
biopsy and resection specimen (Fig 2). Antibiotics
were switched to metronidazole accordingly. The
patient’s condition was later complicated by an
intra-abdominal fluid collection and image guided
drainage was performed. He was initially nursed
in the intensive care unit and then transferred to a
surgical ward for rehabilitation. He was discharged
from hospital 4 months later.
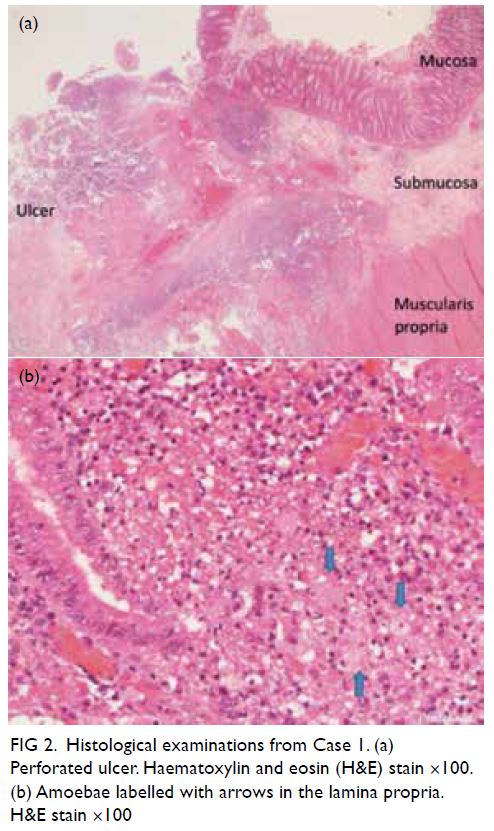
Figure 2. Histological examinations from Case 1. (a) Perforated ulcer. Haematoxylin and eosin (H&E) stain ×100. (b) Amoebae labelled with arrows in the lamina propria. H&E stain ×100
Case 2
In February 2020, a 61-year-old man presented with
a ≥1-week history of diarrhoea, abdominal pain,
high fever of 39°C and tachycardia with normal
blood pressure. Abdominal examination showed
diffuse tenderness, but without peritoneal signs.
The patient’s medical history was otherwise good,
and initial investigations including blood tests and
plain radiographs were all unremarkable. In view
of the progressive abdominal distension, urgent
contrast CT was arranged and showed a suspected
perforated caecum. Emergency surgery found
ischaemic colon extending from the caecum to
mid sigmoid, with perforations over the proximal transverse colon and hepatic flexure. Subtotal
colectomy with end ileostomy was performed.
He was transferred to the intensive care unit after
surgery and required vasopressor and continuous
venovenous haemofiltration due to severe sepsis.
He showed a good response and was weaned
off vasopressor support on postoperative day 4.
Pathology of the surgical specimen later confirmed
amoebic colitis with extensive ulcer and perforation
(Fig 3). There were no features of atherosclerosis,
vasculitis, thromboembolism, or inflammatory
bowel disease. Microscopic results were also
available after surgery. Stool culture for C difficile,
stool microscopy for amoeba, stool microscopy for
ova or cysts, stool polymerase chain reaction for
virus, and HIV serology test results were all negative,
but Entamoeba histolytica serology test was positive.
He was prescribed metronidazole for 14 days and
then oral diloxanide furoate for 10 days as suggested
by the microbiologist. The patient’s recovery
was later complicated by wound dehiscence and
abdominal cocoon. Repeat surgery for debridement
was performed and skin closure changed to an
ABTHERA temporary abdominal closure system.
The patient recovered gradually and was transferred
to a rehabilitation unit 3 months after surgery.
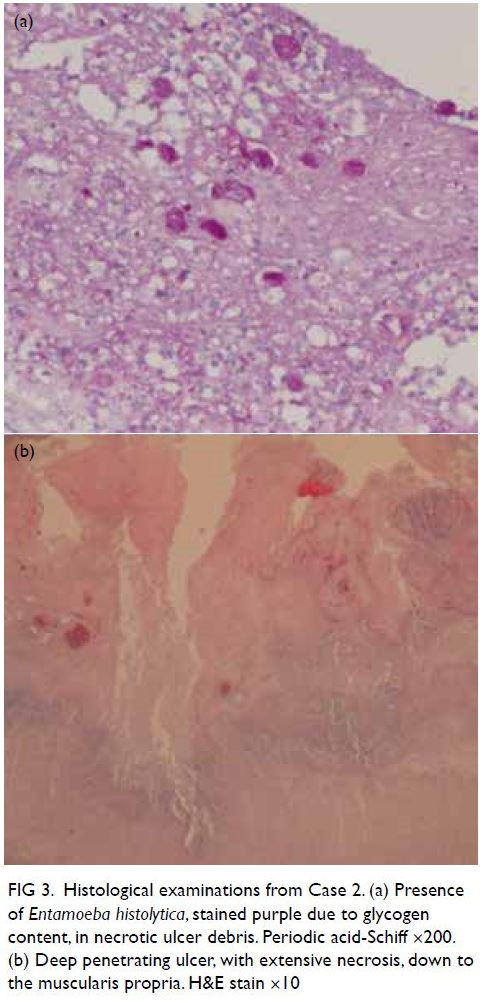
Figure 3. Histological examinations from Case 2. (a) Presence of Entamoeba histolytica, stained purple due to glycogen content, in necrotic ulcer debris. Periodic acid-Schiff ×200. (b) Deep penetrating ulcer, with extensive necrosis, down to the muscularis propria. H&E stain ×10
Discussion
Entamoeba histolytica is a protozoan parasite and
the cause of amoebiasis in humans.1 Early diagnosis
and treatment are essential to avoid progression to
fulminant colitis. Amoebic dysentery is classified
as a notifiable disease in Hong Kong. Prevalence of
amoebiasis is higher in developing countries such
as India, Mexico, and parts of Central and South
America,2 mainly related to poor socioeconomic and
sanitary conditions. In developed countries, where
faecal-oral transmission is unusual, amoebiasis is
more often seen in immigrants from or individuals
with a travel history to endemic areas. Other groups
at risk include male homosexuals3 with or without
HIV, infants, pregnant women, and those taking
immunosuppressants, especially corticosteroids.1
Neither of our two cases had a relevant travel history
in the past year, and they presented several months
apart, so they are unlikely to be imported cases or to have had the same source of infection. Case 1
had some risk factors for amoebiasis: he was a male
homosexual living in rental housing with shared
rooms. A detailed history taking from patients who
present with severe diarrhoea is crucial. Prompt
initiation of anti-amoebic treatment should be
considered in cases where there is a high index of
suspicion.
Although E histolytica can be cultured in vitro, this is neither routinely performed nor is it a gold
standard in the diagnosis of amoebic colitis because
amoebic culture is insensitive (25%). The most
commonly used laboratory test is stool microscopy
to identify trophozoites or cysts, although this also
has low sensitivity (<60%) and specificity (10%-50%).
As exemplified by our two cases, both had negative
stool microscopy. In some laboratories, stool
antigen detection of E histolytica might be available.
However, neither stool microscopy (in the absence
of ingested erythrocytes in the trophozoites) nor
some antigen detection kits can distinguish between
pathogenic E histolytica and commensal Entamoeba
dispar. Serum antibody is commonly positive in
patients with invasive amoebiasis,4 especially in
endemic areas. It may be difficult to differentiate past
from active infection since antibodies may persist for
some time. Recent commercially available multiplex
polymerase chain reaction systems offer a rapid and
sensitive way of detecting E histolytica DNA in stool;
however, cost and availability limit their application
in most patients.
Another popular non-invasive investigation is
different modalities of imaging, especially contrast
CT scan. Some CT features specific to amoebic
colitis have been reported. These include extended
submucosal ulcers with intramural dissection
caused by flask-shaped ulcers typical of amoebiasis
and omental “wrapping” indicating adhesions
with neovascularisation due to ischaemic foci of
transmural amoebic colitis.5 Other non-specific
findings include pancolitis with areas of target signs,
discontinuous bowel necrosis and coexistence of liver
abscess. None of these features were evident on CT
scans in our patients. In clinical practice, CT scan is
not sensitive and has a small role in diagnosing FAC.
It is mainly used to distinguish the severity of colitis,
looking for complications such as bowel perforation
or ischaemia.
Sigmoidoscopy and/or colonoscopy with
biopsy can also be performed as a diagnostic tool.
However, there is a high risk of perforation, especially
of inflamed or even ischaemic bowel. We do not
recommend endoscopic investigations in cases of
severe colitis or in patients with high fever.
Preoperative diagnosis of FAC remains a
challenge and detailed history taking plays an
important role in identifying high-risk cases.
Mild cases of amoebic colitis can be treated
medically with metronidazole to control systemic
invasion and diloxanide furoate, a luminal agent, in
addition to eliminating luminal cysts. If the condition becomes transmural, conservative treatment is no
longer appropriate. Early diagnosis and extensive
surgical treatment are important to reduce
morbidity and mortality.1 In both our cases, with
both complicated by bowel perforation, extensive
bowel resection was immediately performed.
Intra-operatively, skipped lesions with multiple
transmural perforations of bowel were noticed.
However, some gross features make differentiation
from other pathology difficult, especially Crohn’s
disease. In our patients, necrotic tissue was more
friable with little bleeding from the necrotic bowel
wall. These features are quite unique compared
with other causes of bowel ischaemia. This may be
related to severe necrosis with consequent poor
vascular supply to the bowel. In the worst case,
the necrotic bowel wall communicates with the
peritoneal cavity, making bowel resection more
difficult as the dissection planes may be disrupted.
Therefore, extensive debridement of necrotic tissue
or even staged operations are required. Special gross
features of FAC are seldom mentioned in other case
reports. Early identification of these features enables
early commencement of empirical anti-amoebic
treatment and aids the recovery of patients.
Author contributions
Concept or design: LM Tam, KC Ng, CH Man.
Acquisition of data: LM Tam
Analysis or interpretation of data: LM Tam, FY Cheng, Y Gao.
Drafting of the manuscript: LM Tam.
Critical revision of the manuscript for important intellectual content: KC Ng, CH Man.
Acquisition of data: LM Tam
Analysis or interpretation of data: LM Tam, FY Cheng, Y Gao.
Drafting of the manuscript: LM Tam.
Critical revision of the manuscript for important intellectual content: KC Ng, CH Man.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patients were treated in accordance with the Declaration
of Helsinki. The patients provided verbal informed consent
for the treatment/procedures and consent for publication.
References
1. Stanley SL Jr. Amoebiasis. Lancet 2003;361:1025-34. Crossref
2. Haque R, Huston CD, Hughes M, Houpt E, Petri WA Jr. Amebiasis. N Engl J Med 2003;348:1565-73. Crossref
3. Roure S, Valerio L, Soldevila L, et al. Approach to
amoebic colitis: epidemiological, clinical and diagnostic
considerations in a non-endemic context (Barcelona, 2007-2017). PLoS One 2019;14:e0212791. Crossref
4. Tanyuksel M, Petri WA Jr. Laboratory diagnosis of
amebiasis. Clin Microbiol Rev 2003;16:713-29. Crossref
5. Kinoo SM, Ramkelawon VV, Maharajh J, Singh B. Fulminant
amoebic colitis in the era of computed tomography scan:
a case report and review of the literature. SA J Radiol
2018;22:1354. Crossref