Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Gastric peroral endoscopic myotomy for delayed
gastric conduit emptying after pharyngo-laryngo-esophagectomy: a case report
Fion SY Chan, MB, BS, FHKAM (Surgery)1; Ian YH Wong, MB, BS, FHKAM (Surgery)1; Desmond KK Chan, MB, BS, FHKAM (Surgery)1; Claudia LY Wong, MB, BS, FHKAM (Surgery)1; Betty TT Law, MB, BS, FHKAM (Surgery)1; Velda LY Chow, MS, FHKAM (Surgery)2; Simon Law, PhD, MS1
1 Division of Esophageal and Upper Gastrointestinal Surgery, Department of Surgery, School of Clinical Medicine, The University of Hong Kong, Queen Mary Hospital, Hong Kong
2 Division of Head and Neck Surgery, Department of Surgery, School of Clinical Medicine, The University of Hong Kong, Queen Mary Hospital, Hong Kong
Corresponding author: Prof Simon Law (slaw@hku.hk)
Case report
In September 2016, a 64-year-old man with
intrathoracic oesophageal cancer underwent
neoadjuvant chemoradiotherapy and minimally
invasive esophagectomy with no pyloroplasty.
The gastric conduit was placed in the posterior
mediastinum. Pathology revealed a moderately
differentiated squamous cell carcinoma (ypT2N1M0)
with clear margins. One year later he underwent
surgery for isolated right cervical lymph node
recurrence and tolerated a normal diet after surgery
with no gastrointestinal symptoms. At 22 months
after the second surgery, the patient developed
dysphagia and a cervical oesophageal cancer
was identified. Completion pharyngo-laryngo-esophagectomy
(PLE) with resection of the residual
cervical oesophagus, pharyngo-laryngectomy, and
reconstruction with a segment of free jejunum
interposed between the neopharynx and gastric
conduit was performed. After surgery, the patient developed delayed gastric conduit emptying (DGCE)
and reported regurgitation of undigested food soon
after diet introduction. There was a persistently high
nasogastric output, and non-ionic contrast study
showed hold-up of contrast at the level of pylorus
(Fig 1a). The patient’s symptoms persisted and he
relied on nasoduodenal feeding despite prokinetic
agents and pyloric balloon dilatation.
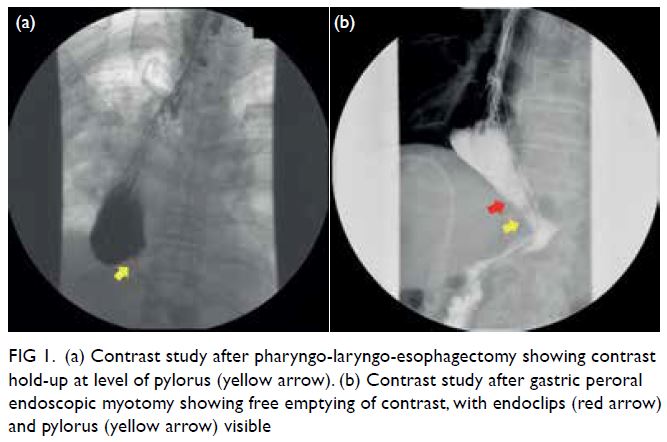
Figure 1. (a) Contrast study after pharyngo-laryngo-esophagectomy showing contrast hold-up at level of pylorus (yellow arrow). (b) Contrast study after gastric peroral endoscopic myotomy showing free emptying of contrast, with endoclips (red arrow) and pylorus (yellow arrow) visible
Gastric peroral endoscopic myotomy
(G-POEM) was performed in February 2020,
4 months after completion PLE. The procedure was
performed with the patient in a supine position
and under general anaesthesia with endotracheal
intubation via end tracheostomy. A high-definition
gastroscope (GIF-H190; Olympus, Tokyo, Japan)
fitted with a conical shaped transparent cap (DH-28GR; Fujifilm, Tokyo, Japan) and carbon dioxide
insufflation were used. After submucosal injection of
a mixture of normal saline and indigo carmine at the
posterior wall of the gastric conduit, 5 cm proximal to
the pylorus, a 2-cm longitudinal mucosal incision was
made with DualKnife J (Olympus) using Endocut Q
mode (effect 3, cut-duration 2, cut-interval 4) [VIO®
300D; Erbe, Tübingen, Germany]. The endoscope
entered the submucosal space to dissect a tunnel
caudally until the pyloric ring was well exposed
(Fig 2a). Pyloromyotomy was performed and the
circular muscle ring completely divided and flattened
(Fig 2b). Haemostasis was achieved and the mucosal
opening closed with repositioning clips (Single Use
Hemoclip; Mednova, Zhejiang, China) [Fig 2c]. The
surgery time was 120 minutes and rapid contrast
passage to the duodenum was demonstrated on
postoperative contrast study (Fig 1b). He resumed
an oral diet thereafter.
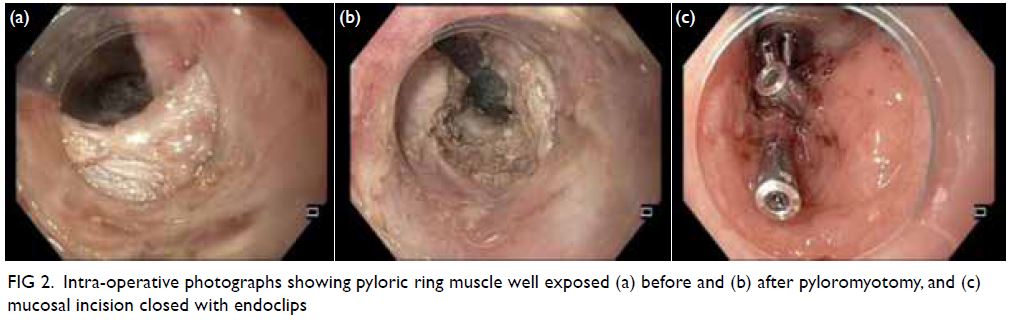
Figure 2. Intra-operative photographs showing pyloric ring muscle well exposed (a) before and (b) after pyloromyotomy, and (c) mucosal incision closed with endoclips
Discussion
Pharyngo-laryngo-esophagectomy was first
reported by Ong and Lee in 19601 and is regarded
as standard treatment for hypopharyngeal and cervical oesophageal cancer. Chemoradiotherapy
has gained popularity as an alternative therapeutic
strategy to preserve the larynx, but salvage PLE
due to incomplete response or cancer recurrence
is not uncommonly required.2 Post-PLE DGCE is
underreported and the incidence is unknown. Patients
frequently complain of bloating, regurgitation, and
poor oral intake. According to unpublished results
from our prospectively collected database, DGCE
was documented in five of 20 patients with PLE for
cervical oesophageal cancer over the past 10 years.
Of these five patients, pyloroplasty was performed in
two, of whom symptoms improved with prokinetic
agents alone in one, and endoscopic pyloric balloon
dilation was required in the other. For those without
pyloric drainage, two patients were managed by
G-POEM. The remaining patient was an 83-year-old
man on prolonged tube feeding who had pneumonia
and died 10 weeks after the surgery.
The pathogenesis of DGCE after PLE may
differ to that after oesophagectomy without
pharyngo-laryngectomy although data are lacking.
Experience in the management of DGCE after
oesophagectomy (without pharyngo-laryngectomy)
serves to guide treatment of post-PLE DGCE.
Proposed contributing factors include gastropyloric
denervation, dysfunctional gastric peristalsis and
use of the whole stomach for reconstruction.3 4
The application of G-POEM in PLE patients
has not been reported. We report a patient with prior
oesophagectomy who developed DGCE only after
completion PLE. Symptoms resolved after G-POEM.
We postulate that removal of the upper oesophageal
sphincter in PLE limits build-up of intragastric
pressure, compounding DGCE. Pyloromyotomy
reduces pyloric channel pressure and expedites
gastric emptying, G-POEM accomplishes this as
a minimally invasive method. We hypothesise that
gastric conduit emptying after PLE can be viewed as
a two-stage process. In the first stage, the food bolus passes passively from the proximal stomach to the
antrum. In the second stage, food is evacuated from
the antrum through the pylorus to the duodenum.
A sufficient pressure gradient within the gastric
conduit is required to overcome pyloric resistance.
Resection of the pharynx and larynx results in
equalisation of pressure between the gastric conduit
and the atmosphere. The stomach is also exposed
to negative intrathoracic pressure. The outflow
resistance due to the intact pylorus assumes more
importance after PLE since the paretic stomach
fails to build up internal pressure. This explains
why symptoms of delayed emptying in our patient
emerged only after the pharyngo-laryngectomy, not
after the initial oesophagectomy.
Pyloroplasty and pyloromyotomy have both
been shown effective and safe drainage procedures
for gastric conduit after oesophagectomy.5 The
G-POEM disrupts the pylorus and improves
gastric emptying, theoretically achieving the same
outcome and serving as a salvage option for DGCE
after PLE. We perform G-POEM according to the
same principle applied to POEM for achalasia. The
submucosal tunnel is dissected close to the muscle
layer for precise pyloromyotomy. Secure mucosal
closure permits early diet resumption. However,
the aim of pyloromyotomy is to overcome the
outlet obstruction without alleviating gastroparesis.
Despite improved gastric emptying, symptoms of
our patient were not completely eliminated. Patients
need to make dietary adjustments to accommodate
the new conduit over time while maintaining
satisfactory nutrition and body weight.
To the best of our knowledge, this is the first
report of successful management of DGCE after
PLE by G-POEM. A pyloric drainage procedure is
advocated since resection of the upper oesophageal
sphincter, an integral part of PLE, limits pressure
build-up and food emptying within the gastric
conduit.
Author contributions
Concept or design: FSY Chan, S Law.
Acquisition of data: FSY Chan.
Analysis or interpretation of data: FSY Chan.
Drafting of the manuscript: FSY Chan.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: FSY Chan.
Analysis or interpretation of data: FSY Chan.
Drafting of the manuscript: FSY Chan.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
As an editor of the journal, VLY Chow was not involved in the peer review process. Other authors have disclosed no
conflicts of interest.
Funding/support
This study received no specific grant from any funding agency
in the public, commercial, or not-for-profit sectors.
Ethics approval
The study was approved by the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong
West Cluster (Ref: UW 16-2023). Consent from patient was
obtained.
References
1. Ong GB, Lee TC. Pharyngogastric anastomosis after
oesophago-pharyngectomy for carcinoma of the
hypopharynx and cervical oesophagus. Br J Surg
1960;48:193-200. Crossref
2. Tong DK, Law S, Kwong DL, Wei WI, Ng RW, Wong KH.
Current management of cervical esophageal cancer. World
J Surg 2011;35:600-7. Crossref
3. Konradsson M, Nilsson M. Delayed emptying of the gastric
conduit after esophagectomy. J Thorac Dis 2019;11:S835-44. Crossref
4. Akkerman RD, Haverkamp L, van Hillegersberg R,
Ruurda JP. Surgical techniques to prevent delayed gastric
emptying after esophagectomy with gastric interposition: a
systematic review. Ann Thorac Surg 2014;98:1512-9. Crossref
5. Law S, Cheung MC, Fok M, Chu KM, Wong J. Pyloroplasty and pyloromyotomy in gastric replacement of the
esophagus after esophagectomy: a randomized controlled
trial. J Am Coll Surg 1997;184:630-6.

