© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Chromosomal abnormalities and neurological outcomes in fetal cerebral ventriculomegaly: a retrospective cohort analysis
WY Lok, FHKAM (Obstetrics and Gynaecology), FHKCOG1; CW Kong, FHKAM (Obstetrics and Gynaecology), FHKCOG1; SYA Hui, FHKAM (Obstetrics and Gynaecology), FHKCOG2; MM Shi, MPhil2; KW Choy, PhD2; WK To, MD, FRCOG1; TY Leung, MD, FRCOG2
1 Department of Obstetrics and Gynaecology, United Christian Hospital, Hong Kong
2 Department of Obstetrics and Gynaecology, The Chinese University of Hong Kong, Hong Kong
Corresponding author: Dr WY Lok (happyah2@hotmail.com)
Abstract
Introduction: This study investigated the incidences
of chromosomal abnormalities and the neurological
outcomes according to the degree of fetal cerebral
ventriculomegaly.
Methods: All women with antenatal ultrasound
diagnosis of fetal cerebral ventriculomegaly were
retrospectively identified from two maternal-fetal
medicine units in Hong Kong from January 2014 to
December 2018. Degrees of fetal ventriculomegaly
were classified as mild (10-11.9 mm), moderate
(12-14.9 mm), or severe (≥15 mm). Genetic
investigation results were reviewed, including
conventional karyotyping and chromosomal
microarray analysis (CMA); correlations between
chromosomal abnormalities and the degree of fetal
ventriculomegaly were explored. The neurological
outcomes of subsequent live births were analysed to
identify factors associated with developmental delay.
Results: Of 84 cases (ie, pregnant women and
their fetuses) included, 46 (54.8%)
exhibited isolated fetal ventriculomegaly, 55 (65.5%)
had mild cerebral ventriculomegaly, and 29 (34.5%)
had moderate or severe cerebral ventriculomegaly.
Overall, 20% (14/70) of cases had chromosomal
abnormalities. Moreover, 12% (3/25) of mild isolated
ventriculomegaly cases had abnormal karyotype or
CMA results. The CMA provided an incremental
diagnostic yield of 8.6% (6/70), compared with conventional karyotyping; 4.3% exhibited pathogenic
variants and 4.3% exhibited variants of uncertain
significance. Among the 53 live births in the cohort,
fewer cases of mild isolated ventriculomegaly were
associated with developmental delay than more
severe isolated ventriculomegaly (9.7% vs 41.7%,
P<0.03).
Conclusions: Chromosomal microarray analysis
testing should be offered to all women with fetal
cerebral ventriculomegaly, including women with
isolated mild ventriculomegaly. The incidence of
developmental delay after birth increases with the
degree of prenatal cerebral ventriculomegaly.
New knowledge added by this study
- All degrees of isolated cerebral ventriculomegaly were associated with chromosomal abnormalities; the incidences of chromosomal abnormalities did not significantly differ according to the degree of ventriculomegaly.
- Chromosomal microarray analysis (CMA) provided an incremental diagnostic yield of 8.6%, compared with conventional karyotyping, for fetal cerebral ventriculomegaly.
- Invasive procedures with CMA testing should be offered to all women with fetal cerebral ventriculomegaly.
- Non-invasive prenatal testing for chromosome abnormalities should not be offered as an alternative to direct invasive genetic testing.
- Women should receive counselling for the neurological outcomes of the children according to the degree of fetal cerebral ventriculomegaly.
Introduction
Assessment of the fetal cerebral lateral ventricle is
a standard requirement during the mid-trimester
morphology ultrasound performed between 18 and
22 weeks of gestation.1 The International Society of Ultrasound in Obstetrics and Gynecology has
recommended a standard method to measure the
size of the lateral ventricle, which should be in an
axial transventricular plane at the atrium of the
posterior horn with calibres placed over the inner edges.2 The reference ranges of lateral ventricle
width were established by Cardoza et al3 in 1988;
they are consistent across gestations. The diameter
(mean ± standard deviation) of the lateral ventricle
is 7.6 ± 0.6 mm (range, 6-9). Therefore, fetal cerebral
ventriculomegaly is defined as dilation of the lateral
ventricle atrium to a width of >10 mm (>4 standard
deviations from the mean).3
The degree of lateral ventricle dilation is
classically categorised as mild (10-11.9 mm),
moderate (12-14.9 mm), or severe (≥15 mm)
for clinical and research purposes. Mild fetal
ventriculomegaly can be isolated and may
represent a normal variant if other pathologies are
excluded.4 Therefore, the identification of cerebral
ventriculomegaly on prenatal ultrasound does
not represent a conclusive diagnosis; it signifies a
need to identify various underlying pathologies,
including structural abnormalities of the central
nervous system (CNS), from hypoxic, haemorrhagic,
infective, and genetic causes. Fetal ventriculomegaly
is considered a marker of abnormal karyotype; it can
be associated with pathogenic copy number variations
(CNVs) identified by chromosomal microarray
analysis (CMA). The Society for Maternal Fetal
Medicine recommends antenatal diagnostic testing
(amniocentesis) with CMA when ventriculomegaly is
detected.4 In this study, we examined the incidences
of abnormal karyotype and CMA results in fetuses with cerebral ventriculomegaly in Hong Kong;
we also evaluated their correlations with different
degrees of ventriculomegaly. We aimed to determine
whether amniocentesis with CMA should be offered
to all fetuses with cerebral ventriculomegaly,
regardless of the degree of ventriculomegaly. We
also reviewed the neurodevelopmental outcomes of
all live births with fetal ventriculomegaly to identify
factors associated with developmental delay.
Methods
This retrospective cohort study included all pregnant
women with antenatal ultrasound diagnosis of fetal
cerebral ventriculomegaly from two maternal-fetal-medicine
units in tertiary referral public obstetric
centres in Hong Kong, United Christian Hospital
and Prince of Wales Hospital, from January 2014
to December 2018. Cases of fetal ventriculomegaly
were identified from the registries of prenatal
ultrasound structural abnormalities, as well as
the antenatal ultrasound and invasive procedures
databases of the respective departments; they were
also identified from the laboratory genetic diagnosis
database of the Chinese University of Hong Kong
(CUHK). All cases of fetal ventriculomegaly in the
two units were carefully analysed by the maternal
fetal medicine specialists, in accordance with
standard departmental protocols. Fetal cerebral
ventriculomegaly was classified as mild (10-11.9 mm),
moderate (12-14.9 mm), or severe (≥15 mm),
according to the greatest atrial width observed
during ultrasound examinations in that pregnancy.
Based on assessments of any associated ultrasound
abnormalities, fetal cerebral ventriculomegaly was
classified as isolated (if cerebral ventriculomegaly
was the only abnormality identified) or non-isolated
(if other structural abnormalities were detected,
including CNS abnormalities of the brain or spine
and abnormalities in other organ systems).
Pregnant women who chose amniocentesis
underwent karyotyping as the standard primary
genetic investigation. Chromosomal microarray
analysis was offered as an additional self-financed
test. The genetic samples of patients from United
Christian Hospital were sent to the Prenatal
Diagnostic Laboratory of Tsan Yuk Hospital; the
genetic samples of patients from Prince of Wales
Hospital were sent to the Prenatal Diagnostic
Genetic Diagnosis Centre of the CUHK. The
microarray platform Perkin Elmer CGX V2.0 (60K
oligonucleotide array) and Affymetrix CytoScan
750K single nucleotide polymorphism array were
used for CMA studies in Tsan Yuk Hospital from
January 2014 to September 2018 and from October to
December 2018, respectively; the Agilent Fetal DNA
chip version 2.0 (8×60k) array comparative genomic
hybridisation and single nucleotide polymorphism
analysis methods were used in the CUHK throughout the study period. The neurodevelopmental outcomes
of live births were reviewed using the hospital’s
computerised clinical management system. Each
child’s development was assessed by a paediatrician
during follow-up; assessments determined the
presence of cognitive impairment, speech delay, fine
and gross motor skills, epilepsy, or developmental
delay.
The study protocol was approved by the
research ethics committees of the respective
hospitals. The Strengthening the Reporting of
Observational Studies in Epidemiology (STROBE)
statement was used as a reporting guideline for this
study. SPSS software (Windows version 20.0; IBM
Corp, Armonk [NY], United States) was used for
data entry and analysis. Comparisons of categorical
variables were performed using the Chi squared test
or Fisher’s exact test, as appropriate. A P value of
<0.05 was considered statistically significant.
Results
From January 2014 to December 2018, there were
55 565 total deliveries in the study centres; 91 fetuses
(0.16%) had antenatal ultrasound diagnosis of
cerebral ventriculomegaly. After the exclusion of
cases (ie, pregnant women and their fetuses) with
incomplete information (eg, incomplete ultrasound
details) and cases that had not delivered in the study
units, 84 cases were included for final analysis. The
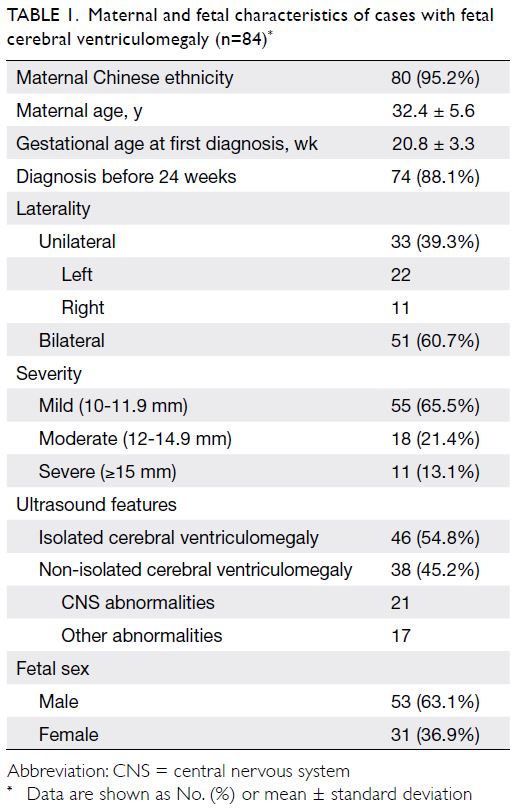
maternal and fetal characteristics are shown in
Table 1. Overall, 65.5% (55/84) of fetuses exhibited
mildly dilated lateral ventricles and 54.8% (46/84) of
fetuses exhibited isolated ventriculomegaly. More
male fetuses had cerebral ventriculomegaly than
did female fetuses (63.1% vs 36.9%). Screening for
congenital fetal infections (eg, cytomegalovirus
in amniotic fluid, maternal blood, or urine;
toxoplasmosis in maternal blood) was conducted in
66.7% of all cases (73.9% of isolated ventriculomegaly
cases); all had negative results. Infection screening
was often not performed in cases of non-isolated
fetal ventriculomegaly associated with other
structural abnormalities; abnormalities in those
cases were often presumed to be associated with
genetic causes, rather than infection. Fetal magnetic
resonance imaging (MRI) was performed in 16 cases
(19.0%) to detect additional CNS abnormalities. The
most common CNS abnormalities associated with
ventriculomegaly were Dandy–Walker malformation
(7 cases), corpus callosum disorders (5 cases), and
spina bifida (3 cases). Other CNS abnormalities
identified included brain tumour, occipital
encephalocele, aqueductal stenosis, lissencephaly,
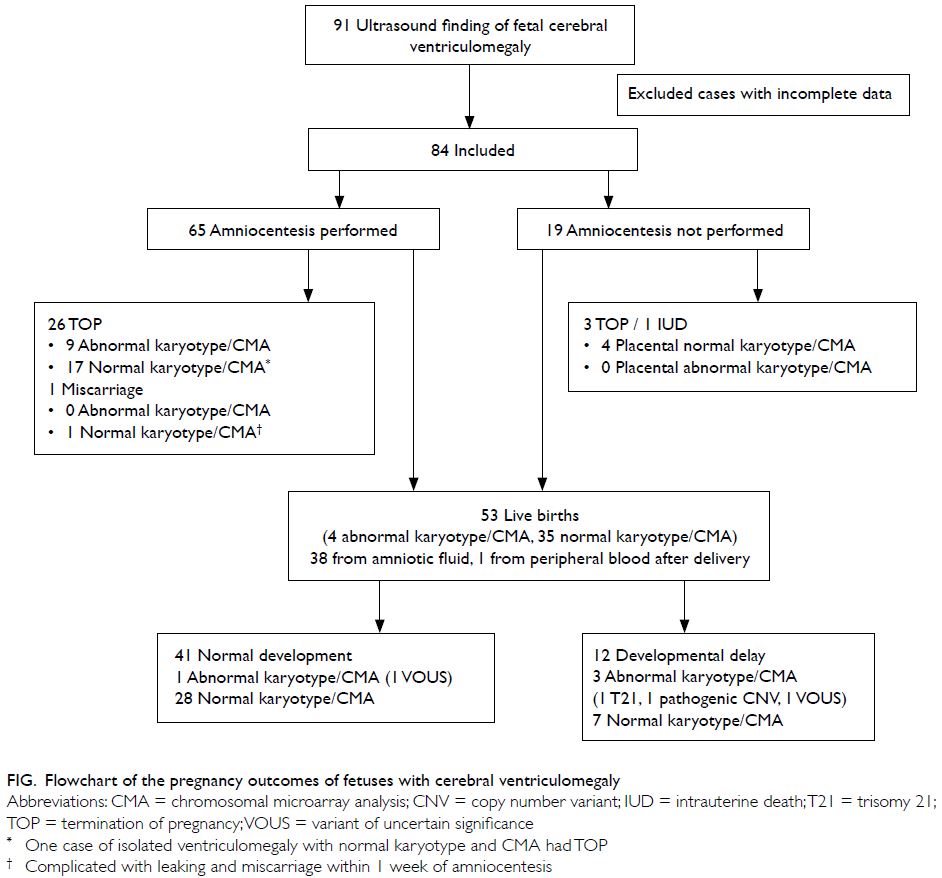
and schizencephaly. The pregnancy outcomes are
shown in the Figure. In total, 53 live births were
delivered in our cohort at a mean gestational age
of 38.1 ± 1.9 weeks. The mean birth weight was
3043 ± 614 g. The mean age at neurodevelopmental outcome assessment of the children was 33 months
(range, 14-72); ultrasound, computed tomography,
or MRI scanning was performed after delivery in
56.6% (30/53) of the cases.
Amniocentesis was performed in 77.4%
(65/84) of cases. Among the 22.6% (19/84) of cases
that did not involve amniocentesis, an invasive
test was declined in 10; in the remaining nine
cases, fetal ventriculomegaly was detected after
24 weeks of gestation, which exceeded the legal
limit for termination of pregnancy in Hong Kong.
The karyotype and CMA results in four cases were
obtained from placental tissue after termination of
pregnancy; in one case, the results were obtained
from the baby’s peripheral blood after delivery.
Altogether, karyotype results were available in
70 cases; CMAs were conducted in 53 of those
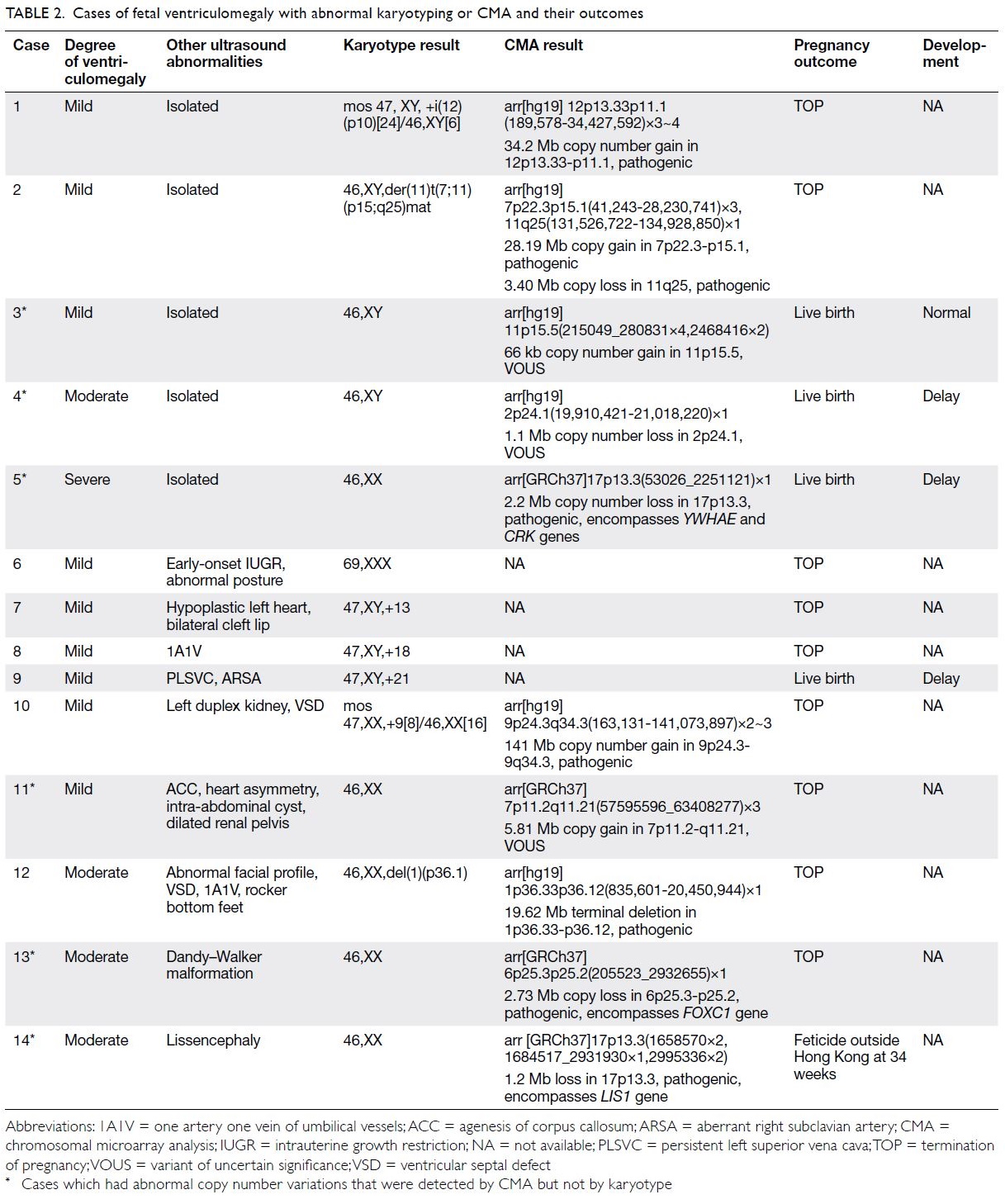
cases. Fourteen cases (20%) had abnormal karyotype
or CMA results (Table 2). In total, 11.4% (8/70) of
cases had chromosomal abnormalities that could
be detected by conventional karyotyping alone,
while six cases (shown in Table 2) had chromosomal
abnormalities that could only be detected by CMA
testing. Therefore, CMA provided an incremental
diagnostic yield of 8.6% (6/70) compared with
conventional karyotyping; three cases exhibited
pathogenic CNVs (4.3%, 3/70) and three cases exhibited variants of uncertain significance (VOUS)
[4.3%, 3/70]. The three pathogenic CNVs included
two cases of 17p13.3 deletion: one involved the
lissencephaly 1 (LIS1) gene and one involved the
YWHAE gene. Deletion of the LIS1 gene has been
associated with classic lissencephaly, microcephaly,
and mental insufficiency; YWHAE may be a
susceptibility gene for schizophrenia.5 The third case
of terminal 6p25 deletion involved the FOXC1 gene,
which is reportedly associated with CNS anomalies
(eg, hydrocephalus and hypoplasia of the cerebellum,
brainstem, and corpus callosum) that cause mild to
moderate developmental delay.6
Subgroup analysis showed that in the isolated
cerebral ventriculomegaly group, 15.2% (5/33) of
cases had abnormal karyotype or CMA results; the
incidences of abnormal karyotype or CMA results
did not significantly differ according to the degree of
isolated cerebral ventriculomegaly (P=0.31) [Table 3]. In the mild isolated ventriculomegaly group, 12.0%
(3/25) of cases had abnormal karyotype or CMA
results, among which two cases could be detected
by conventional karyotyping and one case (VOUS)
could only be detected by CMA.
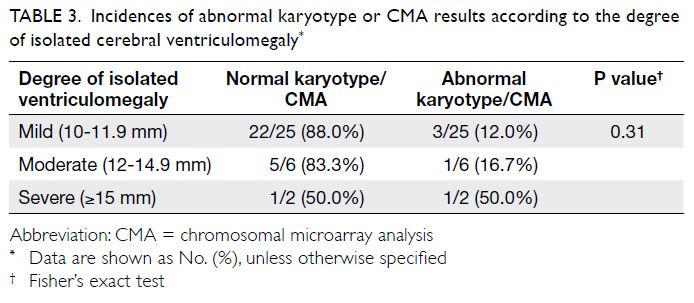
Table 3. Incidences of abnormal karyotype or CMA results according to the degree of isolated cerebral ventriculomegaly
Concerning the evolution of isolated
ventriculomegaly with live births, 8.3% (3/36) with
mild isolated ventriculomegaly and 20% (1/5) with
moderate isolated ventriculomegaly at diagnosis
showed progression during pregnancy. Fewer cases
of mild cerebral ventriculomegaly (10-11.9 mm)
were associated with developmental delay than non-mild
(≥12 mm) ventriculomegaly in the isolated
ventriculomegaly group (9.7% vs 41.7%; P=0.03).
Developmental delay tended to be more common
in the isolated cerebral ventriculomegaly group
with abnormal karyotype or CMA results (66.7% vs
14.8%), compared with isolated ventriculomegaly
with normal karyotype or CMA; however, this difference was not statistically significant. The
risk of developmental delay was not significantly
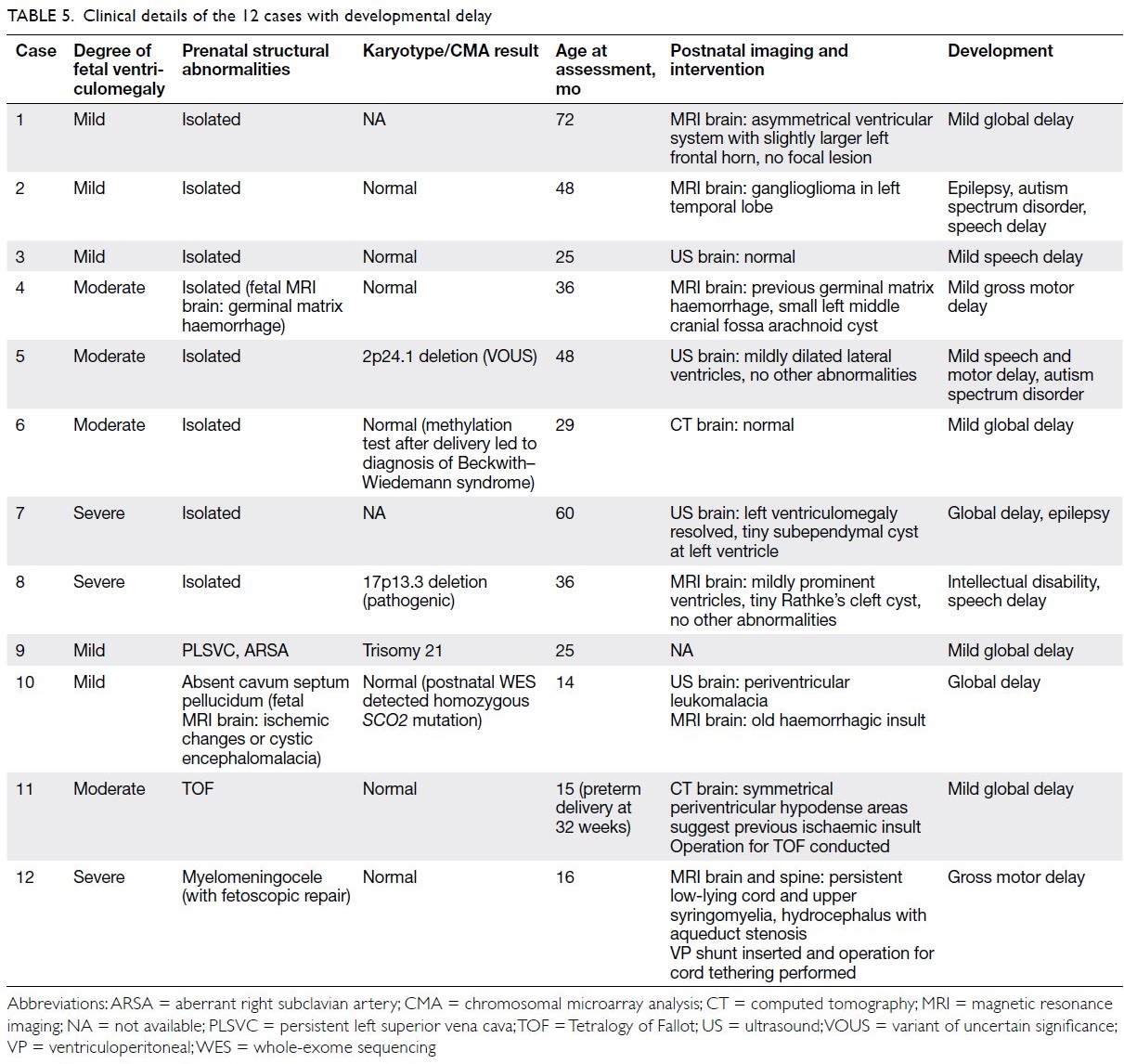
different according to fetal sex in cases of isolated ventriculomegaly (Table 4). The clinical details of the
12 cases with developmental delay are summarised
in Table 5.
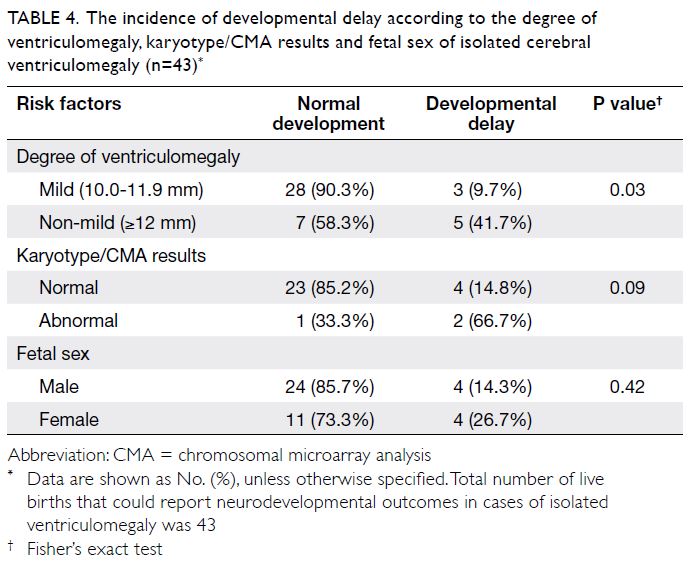
Table 4. The incidence of developmental delay according to the degree of ventriculomegaly, karyotype/CMA results and fetal sex of isolated cerebral ventriculomegaly (n=43)
Discussion
Summary
The incidence of fetal ventriculomegaly in our
cohort was 0.16%, reflecting the incidence of fetal
ventriculomegaly detectable antenatally with mid-trimester
morphology ultrasound examinations
in a large cohort in Hong Kong. Our findings
were compatible with previous reports of fetal
ventriculomegaly incidence, which has ranged from
0.3 to 3.8 per 1000 pregnancies.7 8 While congenital
infection screening was conducted in only 66.7% of
our cases, no cases of intrauterine cytomegalovirus
or toxoplasmosis infection were identified in our
cohort. This is potentially because Chinese pregnant
women have a high cytomegalovirus seroprevalence9
but a low prevalence of toxoplasmosis, compared
with Caucasian pregnant women.10 Because even
mild isolated ventriculomegaly <12 mm carried
a 12.0% risk of chromosomal abnormalities, the findings of amniocentesis with CMA appeared to
be clinically meaningful, regardless of the degree of
fetal ventriculomegaly. In this study, isolated mild
ventriculomegaly was associated with a normal
outcome in approximately 90% of children, but
the risk of developmental delay increased with
increasing degree of ventriculomegaly.
Risk of cerebral ventriculomegaly according
to sex
Cerebral ventriculomegaly was more prevalent in
male fetuses than in female fetuses in our cohort;
the male to female ratio was 1.7. This finding is
consistent with the results of previous studies,
which demonstrated a male predominance
regarding isolated cerebral ventriculomegaly (male
to female ratio of 1.7).11 A study of isolated fetal
ventriculomegaly in China showed no differences
in chromosomal abnormalities between male and
female fetuses (7.6% vs 8.0%, P=0.924).12 Our cohort
demonstrated no significant difference in the risk of
developmental delay according to fetal sex in cases
of isolated ventriculomegaly. Previous studies also
showed no significant differences in neurological
outcomes between male and female infants with
isolated ventriculomegaly and normal karyotype.11
Further studies are needed to explore the reason for
a higher incidence of cerebral ventriculomegaly in
male fetuses than in female fetuses.
Comparison of karyotype and chromosomal
microarray analysis
The incidence of an abnormal karyotype (11.4%
overall vs 8.0% in the mild isolated group) in our
cohort was similar to the results of previous studies.
Previous studies with differences in the proportions
of cases with each degree of ventriculomegaly, as
well as the proportions of associated abnormalities,
demonstrated that the incidence of an abnormal
karyotype in cases of fetal ventriculomegaly was
between 5% and 11.3%.13 14 15 In a systematic review of
isolated ventriculomegaly (10-15 mm), 4.7% (57/1213)
of fetuses had abnormal karyotype results.16 Another
prospective study, which included 355 cases of mild
to moderate ventriculomegaly, showed a higher rate
of abnormal karyotype results when other structural
abnormalities were present (18.0%), compared with
the isolated ventriculomegaly group (10.2%).
Chromosomal microarray analysis testing
provided an incremental diagnostic yield of 8.6%,
compared with conventional karyotyping in our
cohort; 4.3% of cases exhibited pathogenic CNVs,
while 4.3% of cases exhibited VOUS. Chromosomal
microarray analysis can identify aneuploidies (ie,
large structural chromosomal changes); it can also
identify submicroscopic (<5 Mb) CNVs that cannot
be detected by conventional karyotyping.17 Recent studies have focused on the application of CMA
for detecting chromosomal aberrations in cases of
fetal cerebral ventriculomegaly. The incremental
diagnostic yields of CMA over karyotyping for
diagnosing pathogenic CNVs and VOUS in previous
studies of fetal cerebral ventriculomegaly conducted
in China were 3.0% to 12.8% and 2.0% to 4.5%,
respectively.18 19 20 21 A limitation of CMA testing is
the reporting of VOUS, which poses counselling
difficulties during subsequent management. In a
recent cohort in Hong Kong, the rate of VOUS
was 2.1% in prenatal samples obtained for various
indications (eg, abnormal ultrasound, positive Down syndrome screening, abnormal non-invasive
prenatal testing, advanced maternal age, and family
history of chromosomal/genetic disorders).22 Our
cohort detected 4.3% of VOUS, which is high but
generally comparable with the findings of previous
studies.
Consistent with our findings, the incidences
of abnormal karyotype or CMA results in previous
studies did not significantly differ according to the
degree of cerebral ventriculomegaly.23 24 Therefore,
invasive diagnostic tests are warranted for any
degree of cerebral ventriculomegaly identified
in prenatal ultrasound, including mild isolated ventriculomegaly. Chromosomal microarray
analysis should be performed because of its higher
diagnostic yield, compared with conventional
karyotyping. The Hospital Authority of Hong
Kong has replaced conventional karyotyping
with CMA as the primary test for chromosomal
studies of structural abnormalities detected in
prenatal ultrasound since June 2019. Therefore, the
incidences of chromosomal abnormalities detected
in fetal cerebral ventriculomegaly are expected to
increase in the future. Non-invasive prenatal testing
for chromosomal abnormalities by maternal blood
DNA testing is a trend among pregnant women
because of its non-invasiveness. However, non-invasive
prenatal testing for CNVs <5 Mb yielded
a detection rate of only 14.3%.25 The above findings
suggest that non-invasive prenatal testing should
not be offered as an alternative for women with fetal
cerebral ventriculomegaly, regardless of the degree
of ventriculomegaly, because small pathogenic
CNVs can be present in cases that involve any degree
of ventriculomegaly.
Role of genetic mutations in fetal
ventriculomegaly
One of the fetuses in our cohort had mild cerebral
ventriculomegaly; MRI of the brain revealed
ischaemic changes (Table 5 Case 10). Amniocentesis
was performed and showed normal karyotype and
CMA results. The baby had progressive hypertrophic
cardiomyopathy with global developmental delay
after delivery. Trio whole-exome sequencing
(WES) was done after delivery, and the baby was
diagnosed with autosomal recessive mitochondrial
disease caused by SCO2 mutations; both parents
were heterozygous carriers. In prenatal fetal
structural abnormalities, WES can reveal a high
proportion of diagnostic genetic variants, including
up to 22% in CNS abnormalities including cerebral
ventriculomegaly.26 Mutations in two X-linked
genes (L1CAM and AP1S2) and two autosomal
recessive genes (CCDC88C and MPDZ) have been
described to cause congenital hydrocephalus or
aqueductal stenosis, which can cause severe isolated
ventriculomegaly.27 There is a potential role for
WES in facilitating the genetic diagnosis in cerebral
ventriculomegaly with negative karyotype and
CMA results, particularly for those fetuses with
severe ventriculomegaly suggestive of
aqueductal stenosis and in couples with recurrent
fetal abnormalities.
Risk of developmental delay according to the
degree of ventriculomegaly
Fetal cerebral ventriculomegaly was associated with
an increased risk of developmental delay in the child
after delivery. The neurodevelopmental prognosis worsened as the degree of ventriculomegaly
increased in our cohort (9.7% in cases of mild
ventriculomegaly vs 41.7% in cases of moderate
or severe ventriculomegaly) and in other studies.
In a systematic review and meta-analysis of
neurodevelopmental outcomes in cases of
isolated ventriculomegaly (10-15 mm), the overall
prevalence of developmental delay was 7.9%.16 In
a meta-analysis of the neurological outcomes of
fetal ventriculomegaly in China, the neurological
prognosis was good in 88%, 57%, and 36% of mild,
moderate, and severe ventriculomegaly cases,
respectively.13 In another systematic review and
meta-analysis of severe isolated ventriculomegaly,
developmental delay was mild or moderate in 18.6%
of children and severe in 39.6% of children.28 More
than half (58.3%, 7/12) of the children diagnosed
with developmental delay in our study exhibited
only mild delay, although there was a background
risk of mild developmental delay during counselling.
The high incidence of developmental delay in cases
of non-mild isolated ventriculomegaly was probably
also associated with the presence of chromosomal
abnormalities. Nevertheless, our data did not
show associations of abnormal karyotype or CMA
results with developmental delay among the 43 live
births. This finding was presumably biased because
pregnancies were terminated in many of the cases
with abnormal karyotype or CMA results; the
neurological outcomes could not be assessed in
those cases.
Strengths and limitations
This study had some limitations. First, it used
a retrospective cohort design; thus, congenital
infection screening and fetal MRI assessment were
not performed in all cases. Second, there was no
protocol for routine postnatal imaging evaluation,
and the assessment of neurodevelopmental outcomes
among the children was not standardised. However,
our study provided data regarding the incidences of
chromosomal and genetic abnormalities in cases of
antenatally detected fetal ventriculomegaly in Hong
Kong, as well as a general picture of neurological
outcomes of affected children. The findings will allow
prenatal counselling in Hong Kong to be performed
on the basis of more relevant epidemiological and
genomic data, rather than findings from other
populations.
Conclusion
All degrees of cerebral ventriculomegaly may
be associated with chromosomal abnormalities.
Chromosomal microarray analysis has an increased
diagnostic yield, compared with conventional
karyotyping. Amniocentesis with CMA testing
should be offered to all women with fetal cerebral ventriculomegaly. Non-invasive prenatal testing
should not be offered as an alternative method of
chromosomal analysis. The neurological outcomes
of the children are associated with the degree of fetal
ventriculomegaly. Whole-exome sequencing may be
indicated for selected cases of fetal ventriculomegaly
with normal CMA, but further studies are needed to
support this recommendation.
Author contributions
Concept or design: WY Lok, CW Kong, WK To.
Acquisition of data: WY Lok, MM Shi, SYA Hui.
Analysis or interpretation of data: WY Lok, CW Kong, WK To.
Drafting of the manuscript: WY Lok, CW Kong.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: WY Lok, MM Shi, SYA Hui.
Analysis or interpretation of data: WY Lok, CW Kong, WK To.
Drafting of the manuscript: WY Lok, CW Kong.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Ethics approval was obtained from the Kowloon Central/
Kowloon East Research Ethics Committees (Ref: KC/KE-19-0172/ER-4) and The Joint Chinese University of Hong
Kong–New Territories East Cluster Clinical Research Ethics
Committee (CREC Ref No.: 2019.468).
References
1. alomon LJ, Alfirevic Z, Berghella V, et al. Practice guidelines for performance of the routine mid-trimester fetal
ultrasound scan. Ultrasound Obstet Gynecol 2011;37:116-26.Crossref
2. International Society of Ultrasound in Obstetrics & Gynecology Education Committee. Sonographic
examination of the fetal central nervous system: guidelines
for performing the ‘basic examination’ and the ‘fetal
neurosonogram’. Ultrasound Obstet Gynecol 2007;29:109-16. Crossref
3. Cardoza JD, Goldstein RB, Filly RA. Exclusion of fetal
ventriculomegaly with a single measurement: the width
of the lateral ventricular atrium. Radiology 1988;169:711-4. Crossref
4. Society for Maternal-Fetal Medicine (SMFM); Fox NS, Monteagudo A, Kuller JA, Craigo S, Norton ME. Mild fetal
ventriculomegaly: diagnosis, evaluation, and management.
Am J Obstet Gynecol 2018;219:B2-9. Crossref
5. Online Mendelian Inheritance in Man. #247200 Miller-Dieker Lissencephaly Syndrome. Available from: https://www.omim.org/entry/247200. Accessed 1 Aug 2020.
6. Online Mendelian Inheritance in Man. *601090
FORKHEAD BOX C1; FOXC1. Available from: https://www.omim.org/entry/601090. Accessed 1 Aug 2020.
7. Weichert J, Hartge D, Krapp M, Germer U, Gembruch U, Axt-Fliedner R. Prevalence, characteristics and perinatal
outcome of fetal ventriculomegaly in 29,000 pregnancies
followed at a single institution. Fetal Diagn Ther
2010;27:142-8. Crossref
8. Patel SK, Zamorano-Fernandez J, Nagaraj U, Bierbrauer KS, Mangano FT. Not all ventriculomegaly is created equal:
diagnostic overview of fetal, neonatal and pediatric
ventriculomegaly. Childs Nerv Syst 2020;36:1681-96. Crossref
9. Wang S, Wang TZ, Zhang WQ, et al. Cohort study on
maternal cytomegalovirus seroprevalence and prevalence
and clinical manifestations of congenital infection in
China. Medicine (Baltimore) 2017;96:e6007. Crossref
10. Ko RC, Wong FW, Todd D, Lam KC. Prevalence of Toxoplasma gondii antibodies in the Chinese population of
Hong Kong. Trans R Soc Trop Med Hyg 1980;74:351-4. Crossref
11. Melchiorre K, Bhide A, Gika AD, Pilu G, Papageorghiou AT. Counseling in isolated mild fetal ventriculomegaly.
Ultrasound Obstet Gynecol 2009;34:212-24. Crossref
12. Zhao D, Cai A, Wang B, Lu X, Meng L. Presence of chromosomal abnormalities in fetuses with isolated
ventriculomegaly on prenatal ultrasound in China. Mol
Genet Genomic Med 2018;6:1015-20. Crossref
13. Sun Y, Zhang WY. Meta-analysis of fetal lateral ventriculomegaly and prognosis [in Chinese]. Zhonghua
Fu Chan Ke Za Zhi 2018;53:677-82.
14. Gezer C, Ekin A, Ozeren M, et al. Chromosome abnormality
incidence in fetuses with cerebral ventriculomegaly. J
Obstet Gynaecol 2014;34:387-91. Crossref
15. Bijarnia-Mahay S, Puri RD, Kotecha U, et al. Outcome of
prenatally-detected fetal ventriculomegaly. J Fetal Med
2015;2:39-44. Crossref
16. Pagani G, Thilaganathan B, Prefumo F. Neurodevelopmental
outcome in isolated mild fetal ventriculomegaly: systematic
review and meta-analysis. Ultrasound Obstet Gynecol
2014;44:254-60. Crossref
17. Chau MH, Cao Y, Kwok YK, et al. Characteristics and mode of inheritance of pathogenic copy number variants
in prenatal diagnosis. Am J Obstet Gynecol 2019;221:493.
e1-e11. Crossref
18. Duan HL, Zhu XY, Zhu YJ, et al. The application of
chromosomal microarray analysis to the prenatal diagnosis
of isolated mild ventriculomegaly. Taiwan J Obstet Gynecol
2019;58:251-4. Crossref
19. He M, Hu S, Hu T, Zhang Z, Luo H. Correlation between
fetal borderline ventriculomegaly and chromosomal
abnormalities [in Chinese]. Zhonghua Fu Chan Kae Za Zhi
2018;53:660-4.
20. Song TT, Wan SN, LI Y, et al. Application value of
chromosomal microarray analysis in prenatal diagnosis of
lateral ventriculomegaly fetuses [in Chinese]. PLAMJ Med
J Chi People Liberation Army 2017;42:902-8.
21. Li ZZ, Fu F, Lei TY, et al. Application of chromosome microarray analysis for the delineation of pathogenesis for
fetal ventriculomegaly [in Chinese]. Zhonghua Yi Xue Yi
Chuan Xue Za Zhi 2017;34:576-82.
22. Cheng SS, Chan KY, Leung KK, et al. Experience of chromosomal microarray applied in prenatal and postnatal
settings in Hong Kong. Am J Med Genet C Semin Med
Genet 2019;181:196-207. Crossref
23. Zhang ZQ, Xie YJ, Wu JZ, et al. Chromosomal microarray analysis for lateral ventriculomegaly in fetus [in Chinese]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2015;32:789-92.
24. Peng YX, Qiu YW, Chang QX, Yu YH, Zhang M, Li KR.
Clinical value of genome-wide chromosome microarray
technique in diagnosis of fetal cerebral ventriculomegaly
[in Chinese]. Nan Fang Yi Ke Da Xue Xue Bao 2018;38:353-7.
25. Li R, Wan J, Zhang Y, et al. Detection of fetal copy number
variants by non-invasive prenatal testing for common
aneuploidies. Ultrasound Obstet Gynecol 2016;47:53-7. Crossref
26. Petrovski S, Aggarwal V, Giordano JL, et al. Whole-exome sequencing in the evaluation of fetal structural anomalies:
a prospective cohort study. Lancet 2019;393:758-67. Crossref
27. Kousi M, Katsanis N. The genetic basis of hydrocephalus. Annu Rev Neurosci 2016;39:409-35. Crossref
28. Carta S, Agten AK, Belcaro C, Bhide A. Outcome of fetuses with prenatal diagnosis of isolated severe bilateral
ventriculomegaly: systematic review and meta-analysis.
Ultrasound Obstet Gynecol 2018;52:165-73. Crossref