© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Rotational thromboelastometry as a powerful
tool to detect hyperfibrinolysis in a bleeding patient: a case report
KM Kwok, FHKCP, FHKAM (Medicine)1; KL Lee, FHKCP, FHKAM (Medicine)1; SY Lam, FHKCP, FHKAM (Medicine)1; T Liong, FHKCP, FHKAM (Medicine)1; HM Wong, FHKCP, FHKAM (Medicine)1; PY Lam, MBBS, MRCP1; Eudora Y Chow, MBBS, FHKAM (Pathology)2; KI Law, FHKCP, FHKAM (Medicine)1
1 Intensive Care Department, United Christian Hospital, Hong Kong
2 Pathology Department, United Christian Hospital, Hong Kong
Corresponding author: Dr KM Kwok (kkm394@ha.org.hk)
Case report
A 64-year-old man presented to the emergency
department with acute onset abdominal pain. His
medical history was unremarkable. Initial vital
signs showed blood pressure of 87/43 mmHg and
pulse rate of 110 beats per minute. His abdomen
was distended, with generalised tenderness
and guarding. The patient was stabilised with
1 L of crystalloid. Bedside ultrasonographic
examination revealed intraperitoneal free fluid.
Subsequent computed tomography of the abdomen
demonstrated gross haemoperitoneum and multiple
hepatic lesions highly suspicious of hepatocellular
carcinoma (HCC). Active contrast extravasation was
noted at the posterior aspect of segment 2/3 lesion,
compatible with the diagnosis of ruptured HCC. The
initial haemoglobin level was 8.7 g/dL. Transarterial
embolisation was urgently arranged but the patient
went into cardiac arrest. Spontaneous circulation
returned after 8 minutes of cardiopulmonary
resuscitation. Haemoglobin level fell to 5.5 g/dL
and platelet count was normal. Prothrombin
time and activated partial thromboplastin time
were prolonged to 20.3 s and 62.1 s, respectively.
International normalised ratio was 1.9. He was
transfused with 2 units of pack cells and 4 units of
plasma. Transarterial embolisation was successfully
performed. Active contrast extravasation over a
branch of the left hepatic artery was demonstrated
but controlled by Gelfoam injection.
The patient was then transferred to the
intensive care unit. Rotational thromboelastometry
(ROTEM®; Tem International GmbH, Munich,
Germany) was performed to guide transfusion
strategy (Fig a). Maximum lysis was shown to
be 100%, indicating abnormally accelerated clot
lysis. Maximum lysis over 15% is diagnostic of
hyperfibrinolysis. The lysis index at 30 minutes was
5%, indicating almost complete clot dissolution
30 minutes after initial formation. No dysfunction
in coagulation activation or clot propagation was otherwise detected. Tranexamic acid 1 g was
administrated according to the interpretation of
ROTEM results. A follow-up ROTEM analysis after
antifibrinolytic treatment demonstrated restoration
of normal fibrinolysis (Fig b). Haemostasis was
achieved and no further blood transfusion was
needed.
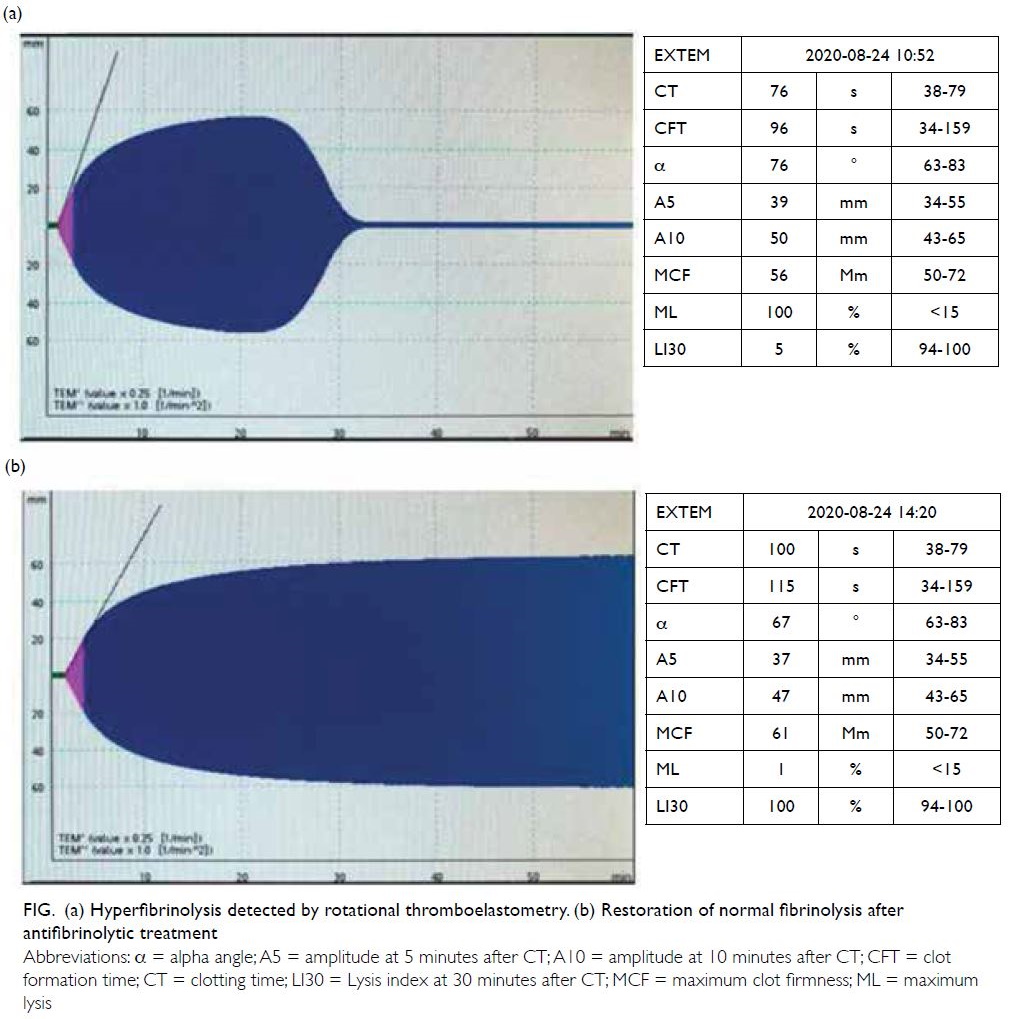
Figure. (a) Hyperfibrinolysis detected by rotational thromboelastometry. (b) Restoration of normal fibrinolysis after antifibrinolytic treatment
Testing for hepatitis B surface antigen was later
revealed to be reactive. This patient probably had liver
cirrhosis and HCC consequent to chronic hepatitis B
infection. The occurrence of ruptured HCC tipped
the balance in this vulnerable patient. He developed
abdominal compartment syndrome and hepatic
failure. The sequential organ failure assessment
score was 13 with an estimated mortality of over
90%. The surgical team advised optimum medical
supportive treatment in view of the extremely high
operative risk. The patient succumbed 16 hours after
hospital admission.
Discussion
Haemocoagulation is a complex interaction of
procoagulants, anticoagulants, fibrinolytic proteins,
and cellular components. Platelets are activated
in response to vascular injury, leading to primary
haemostasis. Activated platelets adhere to damaged
endothelium and aggregate to create a temporary
platelet plug. Secondary haemostasis involves
activation of the coagulation cascade, resulting in
fibrin formation. The platelet plug is strengthened
by the cross-linked fibrin to form a stable clot.
Fibrinolysis serves as the final stage of coagulation to
regulate the extent of clot formation and to maintain
vascular patency. Cross-linked fibrin is broken down
by plasmin and normal blood flow is restored.
Hyperfibrinolysis refers to excessive
fibrinolytic activity that threatens clot integrity
and leads to defective haemostasis. It is common
in patients with chronic liver disease, major
trauma, and obstetric complications. However, the
incidence is not well studied and it has often been underdiagnosed due to an absence of appropriate
tests.1 Conventional coagulation tests, such as
prothrombin time, activated partial thromboplastin
time, and thrombin time, are ineffective in detecting
hyperfibrinolysis. Viscoelastic haemostatic assays are
the only tests for rapid detection and quantification
of hyperfibrinolysis.2
Rotational thromboelastometry is a form of
viscoelastic assay. It provides a global haemostatic
assessment from initial platelet activation, through
platelet aggregation, clot strengthening by cross-linked
fibrin, to clot dissolution. The degree of clot
lysis is expressed numerically as maximum lysis.
Hyperfibrinolysis is diagnosed when maximum lysis
exceeds 15% within a 60-minute ROTEM analysis.
Schöchl et al3 further quantified this condition based on the time course of clot dissolution, as reflected by
the lysis index at 30 minutes and at 60 minutes. They
categorised complete clot lysis within 30 minutes
as fulminant hyperfibrinolysis; intermediate when
complete clot lysis was within 30 to 60 minutes; and
late when complete clot lysis exceeded 60 minutes.
Prompt recognition of hyperfibrinolysis by ROTEM
analysis guides appropriate antifibrinolytic therapy.
Untreated hyperfibrinolysis has been shown to be
associated with refractory bleeding and increased
mortality.1
Rotational thromboelastometry has multiple
advantages over conventional coagulation tests.
In contrast to conventional coagulation tests that
use plasma, ROTEM uses whole blood and can
determine the contribution of both cellular and plasma components of haemostasis. Conventional
coagulation tests provide only a quantitative
assessment of individual procoagulation factors;
results are not necessarily a good indication of
the in vivo haemostatic process, especially in
patients with chronic liver disease.4 Rotational
thromboelastometry also provides real-time
functional assessment of the haemostatic process,
from clot formation to its lysis, and is useful for
identifying hyperfibrinolysis. There is evidence
that use of ROTEM-guided haemostatic strategy
reduces transfusion requirement in different clinical
settings.5 6 7
Understanding its limitations is equally
important in the interpretation of ROTEM analysis.
It is insensitive to the effect of platelet inhibitors
such as aspirin and clopidogrel. Thrombin, the
strongest activator of platelets, is produced in large
amounts during ROTEM analysis, masking the
inhibitory effects of antiplatelet agents.8 It is also
poor at detecting conditions that affect platelet
adhesion and aggregation, with Von Willebrand’s
disease being a well-known example.9 The ROTEM
analysis is undertaken at 37°C to mimic physiological
conditions so the negative effect of hypothermia on
coagulation is not reflected by the analysis.9
In conclusion, owing to the limitations of
conventional coagulation tests, hyperfibrinolysis is
frequently underdiagnosed. With its ability of real-time
functional haemostatic assessment from clot
formation to lysis, ROTEM analysis allows better
insight into the complex haemocoagulation process.
The rapid detection of hyperfibrinolysis by ROTEM
can guide prompt antifibrinolytic treatment, possibly
reducing the transfusion requirement in bleeding
patients.
Author contributions
Concept or design: KM Kwok, KL Lee, SY Lam, T Liong, KI Law.
Acquisition of data: KM Kwok.
Analysis or interpretation of data: KM Kwok, KL Lee, SY Lam, T Liong, KI Law.
Drafting of the manuscript: KM Kwok.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: KM Kwok.
Analysis or interpretation of data: KM Kwok, KL Lee, SY Lam, T Liong, KI Law.
Drafting of the manuscript: KM Kwok.
Critical revision of the manuscript for important intellectual content: All authors.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki. Patient was incapable to provide informed consent for publication.
References
1. Theusinger OM, Wanner GA, Emmert MY, et al. Hyperfibrinolysis diagnosed by rotational
thromboelastometry (ROTEM) is associated with higher
mortality in patients with severe trauma. Anesth Analg
2011;113:1003-12. Crossref
2. Yeung MC, Tong SY, Tong PY, Cheung BH, Ng JY,
Leung GK. Use of viscoelastic haemostatic assay in
emergency and elective surgery. Hong Kong Med J
2015;21:45-51. Crossref
3. Schöchl H, Frietsch T, Pavelka M, Jámbor C.
Hyperfibrinolysis after major trauma: differential diagnosis
of lysis patterns and prognostic value of thrombelastometry.
J Trauma 2009;67:125-31. Crossref
4. Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med 2011;365:147-56. Crossref
5. De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography-guided blood product use before
invasive procedures in cirrhosis with severe coagulopathy:
a randomized, controlled trial. Hepatology 2016;63:566-73. Crossref
6. Veigas PV, Callum J, Rizoli S, Nascimento B, da Luz LT.
A systematic review on the rotational thrombelastometry
(ROTEM®) values for the diagnosis of coagulopathy,
prediction and guidance of blood transfusion and
prediction of mortality in trauma patients. Scand J Trauma
Resusc Emerg Med 2016;24:114. Crossref
7. Vymazal T, Astraverkhava M, Durila M. Rotational
thromboelastometry helps to reduce blood product
consumption in critically ill patients during small surgical
procedures at the intensive care unit—a retrospective
clinical analysis and literature search. Transfus Med
Hemother 2018;45:385-7. Crossref
8. Lang T, von Depka M. Possibilities and limitations
of thrombelastometry/-graphy [in German].
Hamostaseologie 2006;26(3 Suppl 1):S20-9. Crossref
9. Srivastava A, Kelleher A. Point-of-care coagulation testing. Continuing Educ Anaesthesia Crit Care Pain 2013;13:12-6. Crossref

