Hong Kong Med J 2021 Aug;27(4):306–8 | Epub 11 Aug 2021
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
COMMENTARY
Preventing the spread of COVID-19 from chest drains
PY Lung, RN, MSc; SY Au, MB, BS, FHKAM (Medicine); KM Fong, MB, ChB, FHKAM (Medicine); CH Yeung, RN, MSc;
Ruby HM Wong, MSc, FHKAN; YN Tsang, MSc, FHKAN; George WY Ng, MB, BS, FHKAM (Medicine)
Department of Intensive Care, Queen Elizabeth Hospital, Hong Kong
Corresponding author: Dr SY Au (h0145237@gmail.com)
Introduction
For a patient with coronavirus disease 2019
(COVID-19) with chest drain inserted for
pneumothorax, air leakage from the chest drain
system, which is not a closed system, can aerosolise
when bubbling through the underwater seal and
cause viral spreading.1 There are limited reports
suggesting means to prevent viral spread by
modifying the chest drain systems for patients
with COVID-19.2 The chest drain system used in
our unit is the Atrium Ocean (MAQUET Medical
Systems, United States). This system has three main
chambers—a fluid collection chamber, a chamber
with water-seal to monitor degree of air-leak, and
a suction control chamber. The safety port valve,
which is open to the air, prevents the chest drain
system from forming a closed system which might
in turn potentially cause tension pneumothorax,
especially when suction is not applied. This safety
port valve however creates a potential pathway for
viral spread from the drainage system. Herein, we
suggest modifications to the Atrium chest drain
system and describe the subsequent COVID-19 test
results from various parts of the system to test the
effectiveness of the modifications.
Chest drain system modifications
We suggest three modifications on the system
(Figure 1).

Figure 1. A modified Atrium Ocean (MAQUET Medical Systems, United States) chest drain system. (A) Cook adapter and bacterial viral filter, (B) 0.05% chlorhexidine gluconate solution to replace the water in the water seal chamber, and (C) water added in suction control chamber
0.05% Aqueous-based chlorhexidine
gluconate solution in water seal chamber
Kumar et al3 suggested the use of 1% sodium
hypochlorite solution (ie, bleach) to replace the
water in the water seal chamber for patients with
COVID-19. However, we suggested the use of 0.05%
aqueous-based chlorhexidine gluconate solution, to
mitigate the potential risk of chlorine gas leaking
back through the system to the patient. This 0.05%
chlorhexidine gluconate solution is as effective
as povidone-iodine or 70% ethyl alcohol against
severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2).4
Water column in the suction control
chamber to water seal the chamber
Sterile water (5 mL) was added to the suction control chamber to water seal it, so that excessive gas could
not escape the chest drain system through the suction
control port. Any excessive air leak to this chamber,
if any, could only leak out through the suction port.
Bacterial viral filter at the suction port
A bacterial viral filter (BVF) has a filtration
efficiency rate of >99.9999% as tested with particle
size of around 3 μm, which is effectively the size of SARS-CoV-2. The filter has a small dead space
that minimises rebreathing of carbon dioxide. To
connect the BVF to the suction port, the suction
control stopcock is disconnected from the suction
port. An adapter with diameter 15 mm from the
Cook Airway Exchange (Cook Medical) is then used
to connect the suction port to the BVF. If the Cook
Airway Exchange adaptor is not available, an adaptor
of a 7.5-Fr endotracheal tube is an alternative. The
length of the suction port should be such that the
filter does not touch the floor, or it might create a
closed chest drain system, potentially increasing the
risk of tension pneumothorax.
Practical experience
Sample collection
The above modified system was connected to a
patient with COVID-19 who required mechanical
ventilation for his chest infection complicated
by pneumothorax. Several swabs from the chest
drain system were taken and tested by polymerase
chain reaction (PCR) for COVID-19. Swabs were
taken from four sites on of the chest drain system
(Fig 2). Swabs were taken with the sampling sticks moistened by sterile water. The sampling sticks were rolled over the sample areas at least 10 times
in a circular motion. The swabs were sent to the
microbiology laboratory immediately for testing
for SARS-CoV-2 using a reverse transcription PCR
(RT-PCR) TIB-E-gene test (TIB Molbiol
Syntheselabor GmbH, Berlin, Germany) with the
corresponding cycle threshold (CT) value. For Site 1,
the chest drain catheter was disconnected from the
tubing of the chest drain system and the swab was
taken from the inside of the tubing. For Site 2, 1 mL
of fluid from water seal chamber was aspirated. For
Site 3, the tubing was disconnected from the BVF
and the inside of the tubing was swabbed. For Site 4,
the surface of the area beyond the BVF was swabbed.
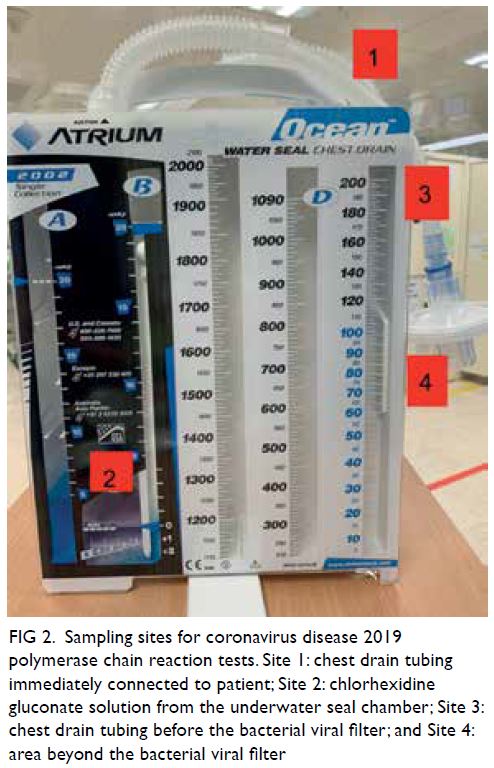
Figure 2. Sampling sites for coronavirus disease 2019 polymerase chain reaction tests. Site 1: chest drain tubing immediately connected to patient; Site 2: chlorhexidine gluconate solution from the underwater seal chamber; Site 3: chest drain tubing before the bacterial viral filter; and Site 4: area beyond the bacterial viral filter
Test results
For Site 1, testing with SARS-CoV-2 RT-PCR
TIB-E-gene was positive and the CT value was
29.51, compared with the CT value of 16.1 from the
tracheal aspirate sample taken in the same patient on
the same day. For Sites 2 to 4, SARS-CoV-2 RT-PCR
TIB-E-gene tests were all negative.
Discussion
The results suggested the potential effectiveness
of our system. Firstly, COVID-19 was detected in
Site 1 (the chest drain catheter), confirming that
the virus can potentially spread from a chest drain.
Secondly, COVID-19 was not detected in Site 2,
suggesting that the 0.05% chlorhexidine gluconate
solution was effective against SARS-CoV-2. This is
further supported by the negative COVID-19 result
in Site 3. Because the virus was not detected in Site
3, the negative result in Site 4 does not necessarily
demonstrate the efficacy of the BVF in preventing
the spread of COVID-19. Nevertheless, although the
efficacy of individual modification of the chest drain
system could not be demonstrated, the complete
system proved to be effective in isolating the virus in
the chest drain system.
The number of patients with COVID-19 in
Hong Kong has been small, and pneumothorax
is not a common presentation for patients with
COVID-19. To validate the efficacy of our suggested
modifications, further testing of the system is
required.
Simple wall suction applied to the system
could provide even better safeguard. However,
suction is not clinically indicated for all patients
with pneumothorax. Furthermore, wall suction
cannot be applied during transport of the patient.
If suction is clinically indicated, the modified chest
drain system can be connected to the wall suction
in the usual manner after removing the BVF; the
BVF is unnecessary after connection to wall suction
because the system is now closed.
Conclusion
Our proposed modifications of the chest drain
system could potentially prevent the spread of
COVID-19 in patients with pneumothorax. Further
study is required to confirm the safety and efficacy of
these modifications.
Author contributions
All authors contributed to the concept or design of the study, acquisition of the data, analysis or interpretation of the
data, drafting of the manuscript, and critical revision of the
manuscript for important intellectual content. All authors
had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Ethics approval
Research ethics application was waived for this study by the KC/KE Research Ethics Committee. Verbal consent was taken from the patient’s relatives. The study conformed to
the ethical guidelines of the 1975 Declaration of Helsinki
as reflected in a priori approval by the institution’s human
research committee.
Availability of data and materials
The data presented in the article can be obtained for studies with aim to test the efficacy of preventing COVID-19 spread
by modification of the chest drain system by contacting the
corresponding author by email at h0145237@gmail.com
within 3 years after this article is published.
References
1. Duffy C, Kidd A, Francis S, et al. Chest drain aerosol generation in COVID-19 and emission reduction
using a simple anti-viral filter. BMJ Open Respir Res
2020;7:e000710. Crossref
2. Bilkhu R, Viviano A, Saftic I, Bille A. COVID-19: Chest
drains with air leak—the silent ‘super spreader’? Available
from: https://www.ctsnet.org/article/covid-19-chest-drains-air-leak-%E2%80%93-silent-%E2%80%98super-spreader%E2%80%99. Accessed 28 Sep 2020.
3. Kumar N, Kumar A, Kumar A, Kumar S. Coronavirus Disease–2019: modified underwater seal chest drain
system. J Cardiothoracic Vasc Anesth 2021;35:347-8. Crossref
4. Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation
with biocidal agents. J Hosp Infect 2020;104:246-51. Crossref

