© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PICTORIAL MEDICINE
Picture-in-picture video demonstration of
systematic transperineal prostate biopsy
KL Lo, FHKAM (Surgery); David Leung, MRCS; Zoe Lai, RN; Crystal Li, RN; SF Ma, MB, ChB; Julius Wong, MRCS; KK Yuen, FHKAM (Surgery); Joseph Li, FHKAM (Surgery); Peter Chiu, FHKAM (Surgery);
SK Mak, FHKAM (Surgery); Joseph Wong, FHKAM (Surgery); CF Ng, FHKAM (Surgery)
Department of Surgery, The Chinese University of Hong Kong, Hong Kong
Corresponding author: Dr KL Lo (lokalun@surgery.cuhk.edu.hk)
 A video clip demonstrating systematic transperineal prostate biopsy is avaialble at www.hkmj.org
A video clip demonstrating systematic transperineal prostate biopsy is avaialble at www.hkmj.orgWe first performed 10-core transperineal prostate
biopsy at our institution in 2018. After having
acquired the technique and experience, we
developed a biopsy protocol that could be modified
according to prostate size (Fig): 10 cores for prostate
size <30 cc, 16 cores for prostate size 30 to 50 cc,
and 20 cores for prostate size >50 cc.
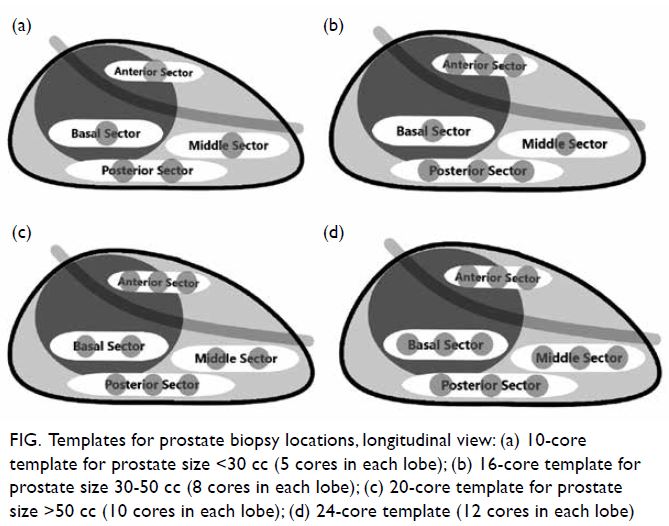
Figure. Templates for prostate biopsy locations, longitudinal view: (a) 10-core template for prostate size <30 cc (5 cores in each lobe); (b) 16-core template for prostate size 30-50 cc (8 cores in each lobe); (c) 20-core template for prostate size >50 cc (10 cores in each lobe); (d) 24-core template (12 cores in each lobe)
We currently take a 24-core prostate biopsy
(Fig d), irrespective of prostate size, to further
improve the detection of prostate cancer. There is
no gold standard protocol for total number of cores
required in any patient; it is dictated by hospital
policy, availability of resources, and the experience
of the urologist.
This video demonstrates systematic
transperineal prostate biopsy.
The instruments used during the procedure
comprised an ultrasound machine with long side-fire
sensor probe, a bed with two leg supports to
facilitate lithotomy position, one disposable biopsy
gun, two needles for local anaesthesia, two metal
trocars, and eight specimen bottles.
Before the procedure and in the absence
of any contraindication, patients are prescribed
a single dose per oral 1 g Augmentin and 500 mg
ciprofloxacin. One tube of per rectal 4.5 oz Fleet
Enema is administered as bowel preparation. Local anaesthetic (EMLA) cream is applied to the patient’s
perineum 1 hour before the procedure.
Step 1: the perineum is disinfected with
chlorhexidine.
Step 2: 10 mL of 1% lidocaine is injected
through each side of the perineum into the
periprostatic plane as local anaesthesia, at an angle
of 45° and 15 mm away from the anus as shown.
It is vital to maintain the needle parallel to the ultrasound probe to ensure continued visualisation
of the needle.
Step 3: a 14-gauge metal trocar is inserted
through the right side of the perineum under
ultrasound guidance.
Step 4: anterior sector prostate biopsies are
taken by tilting an 18-gauge disposable biopsy gun
downwards.
It is important not to hit the urethra during
the procedure.
Step 5: basal sector prostate biopsies are taken.
Step 6: central sector prostate biopsies are
taken.
Step 7: posterior sector prostate biopsies are
taken.
Step 8: Left lobe prostate biopsies are also
taken using a technique similar to that for the right lobe.
Final step: After removing the metal trocars, haemostasis is achieved by compression. A
transparent adhesive film dressing is sprayed over
the two puncture sites.
No post-procedure antibiotic is required.
Author contributions
All authors contributed to the concept or design of the study,
acquisition of the data, analysis or interpretation of the
data, drafting of the manuscript, and critical revision of the
manuscript for important intellectual content. All authors
had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research
Ethics Committee (Ref CREC 2018.323). The patient provided written informed consent.

