© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Anaphylaxis after surgical excision of
subcutaneous infection with parasitic
Dirofilaria: a case report
Brian WC Luk, MB, BS; CN Cheung, MB, ChB, FHKAM (Orthopaedic Surgery); YF Chan, MB, BS, FHKAM (Orthopaedic Surgery)
Department of Orthopaedics and Traumatology, Pok Oi Hospital, Hong Kong
Corresponding author: Dr Brian WC Luk (lukwingchung@gmail.com)
Case report
In January 2018, a 21-year-old man with good past health presented with a 2-week history of left forearm
painless lump. He had no fever. The lump was 30 mm
in diameter with no evidence of inflammation.
Preoperative diagnosis was a sebaceous cyst and preoperative blood tests were not routinely performed.
Surgical excision was performed under local
anaesthesia with lidocaine and application of a
tourniquet. Intra-operatively, a whitish-yellowish
25-mm subcutaneous nodule surrounded by dense
adhesions without a definite border was removed.
The nodule was firm and multi-lobulated with
multiple feeding vessels. Although en bloc excision
with a 5-mm margin was attempted, the dense fibrous
mass was partially breached during dissection due to
scarring.
After tourniquet release, the patient developed
flushing, dizziness, diarrhoea, hypotension, and
sinus tachycardia. He had no respiratory distress but
the clinical diagnosis was anaphylactic shock. He was
stabilised with fluid resuscitation and intravenous
adrenaline. Laboratory tests showed an elevated
white blood cell count (WBC) at 13.6 × 109/L
(reference range: 3.9-10.7 × 109/L), neutrophil
predominance at 85.9% (reference: 38%-76%),
and low eosinophil count of only 0.027 × 109/L
(reference: <0.45 × 109/L) and 0.2% of total WBC.
Blood tests were otherwise unremarkable. Serum
mast cell tryptase level was 46.2 μg/L immediately
after surgery but dropped to 3.3 μg/L after 24 hours.
Erythrocyte sedimentation rate or C-reactive protein
were not measured. The patient remained well and
was discharged home the next day.
The patient had no known history of allergy
and skin allergy test for exposed agents including
lidocaine was negative. On further questioning, he
revealed regular contact with horses located in a
countryside barn but no contact with other animals.
He reported skin erythema (Fig 1) prior to swelling
onset but had presumed this was due to a mosquito
bite.
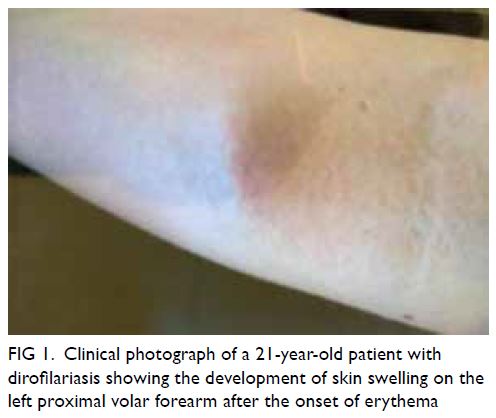
Figure 1. Clinical photograph of a 21-year-old patient with dirofilariasis showing the development of skin swelling on the left proximal volar forearm after the onset of erythema
Microscopy of the nodule revealed a piece
of fibro-fatty tissue with mixed inflammatory cell infiltrate and dense eosinophilic infiltrate (Fig 2a),
and a 0.72 mm × 0.29 mm fragment of degenerated
parasite rimmed by foamy histiocytes (Fig 2b).
There was no evidence of malignancy. The surgical
margin measured from the edge of the surrounding
granulomatous inflammation to the closest edge
of the excised specimen was 0.82 mm. Further
molecular study by polymerase chain reaction and
DNA sequencing based on Filarioidea cytochrome
oxidase subunit I (cox1) and specific 12S ribosomal
RNA (12S rRNA) gene revealed the parasite to be
Dirofilaria hongkongensis.1
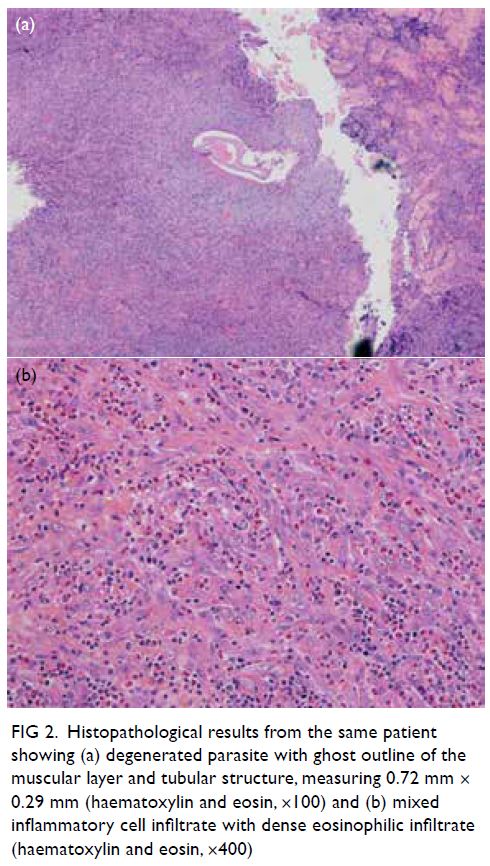
Figure 2. Histopathological results from the same patient showing (a) degenerated parasite with ghost outline of the muscular layer and tubular structure, measuring 0.72 mm × 0.29 mm (haematoxylin and eosin, ×100) and (b) mixed inflammatory cell infiltrate with dense eosinophilic infiltrate (haematoxylin and eosin, ×400)
The patient remained symptom-free and
differential WBC and C-reactive protein were
normal at 6 months after surgery. No antiparasitic
therapy was deemed necessary by microbiologists.
Discussion
Dirofilaria hongkongensis has been proposed as a novel species causing zoonotic filariasis in humans
and is a possible cause of unresolved subcutaneous
nodules in Hong Kong.2 This is the first reported case
worldwide of anaphylactic shock following excision
of subcutaneous dirofilariasis in a human.
Zoonotic filariae are transmitted to humans
through the bite of an infected arthropod such
as mosquitoes. However, they cannot grow to
maturity in accidental hosts such as humans.3 The
pathogenesis is localised foreign body reaction
around a moribund parasite. The absence of a host
inflammatory response in the asymptomatic period
suggests death of the worm due to an unfavourable
host environment, rather than host immunity.3
Asymptomatic survival and growth of the parasite
may continue for ≥6 months. The lesion becomes
clinically noticeable due to granulomatous reaction
with tissue scarring and can present as a subcutaneous
or ocular lesion, and rarely as lymphadenopathy and
nodules in deeper tissues such as the lungs. In our
patient, the subcutaneous dirofilariasis presented as a
painless lump in his forearm, without any symptoms
or signs of inflammation. The patient reported some
skin erythema prior to the onset of the swelling. The
erythema could have been caused by a mosquito bite, which is a possible route of parasite transmission.
The absence of pain, warmth, or erythema over the
mass would suggest the parasite did not trigger a
significant inflammatory response in our patient.
After the parasite’s death, the remaining material
could be shielded off from the host tissue by a dense
fibrous tissue envelope, producing a lump which was
otherwise asymptomatic.
Careful history taking may reveal exposure
to animals. Subcutaneous infections are small
(0.5-1.5 cm) and discrete. Pain or sense of a moving
worm may be present. Blood tests for eosinophilia
and elevated inflammatory markers might be useful,
but the absence of systemic inflammation is common3
and blood test results may be unremarkable. In our
patient, blood tests were not taken preoperatively, as
the clinical impression of the mass was a sebaceous
cyst, with the absence of signs of inflammation.
Postoperative blood tests showed neutrophilia
instead of eosinophilia, but the results were likely
affected by the anaphylaxis.
Among around 40 species of Dirofilaria,
D immitis and D repens account for most cases
of infection in humans. Dirofilaria immitis is
commonly known as “dog heartworm” and has
a cosmopolitan distribution. Hou et al4 reported
seropositivity in 30.6% of stray dogs and 15.6% of
domestic dogs in north-eastern China. Wang et al5
reported a 0% to 7.4% seroprevalence in dogs in
coastal cities in south-eastern China. Dirofilaria
repens is prevalent worldwide including Southeast
Asia. Dirofilaria hongkongensis was first proposed as
a distinct species in 2012, following three cases of
human infection.2 In stray dogs in Hong Kong, the
seroprevalence of D hongkongensis is 3%,2 and that of
D immitis is 10%.6
Molecular study in nucleotide sequencing of cytochrome oxidase subunit 1 (cox1) gene and the
12S ribosomal RNA (12S rRNA) gene is useful in the identification of Dirofilaria species, taking reference
from GenBank data. The cox1 gene and 12S rRNA
gene specific to D hongkongensis were identified
(GenBank accession number NC_031365). Simpler
diagnostic tests would be less reliable; for example,
morphological identification depends on the quality
of histopathological specimen, and in our case
only parasitic fragments were found. Retrospective
molecular study could also be performed on the
stored specimen for epidemiological studies.
Parasitic materials are foreign antigens that
may trigger a type I immunoglobulin E–mediated
hypersensitivity reaction. Dirofilaria immitis extract
can result in shock and elevated plasma histamine
level in dogs,7 while hydatid cyst (Echinococcus
spp) rupture has been associated with anaphylactic
shock.8 We believe the partial breach of the parasitic
tissue envelope during our surgical dissection led to
contact of parasitic material with host tissue. This
contact, in turn, caused the hypersensitivity reaction
and anaphylactic shock in our patient.
We recommend complete en bloc excision
of lesions suspected to be caused by dirofilariasis
to prevent anaphylaxis, especially when surgeons
encounter dense adhesions or multiple feeding
vessels. Further study is required to ascertain the
necessary margin of excision to avoid inadvertent
breakage of the tissue envelope. Up to 75.4% of
parasitised humans experience chronic urticaria.9
In our patient, the capsule was breached during
dissection and sudden allergen release may have
triggered the anaphylactic cascade. Antiparasitic
medication is likely unnecessary if the parasite can
be removed intact.
Anaphylactic shock can cause sudden
haemodynamic collapse. It is characterised by acute
onset of hypotension after allergen exposure, or
the combination of cutaneous, cardiopulmonary or
gastrointestinal manifestations.10 The importance
of routine monitoring, timely detection and
cardiopulmonary stabilisation cannot be
overemphasised. Plasma tryptase or histamine
level may serve as a diagnostic adjunct in doubtful
cases. Fluid resuscitation, supplemental oxygen,
and epinephrine injection are indicated as effective
treatments of anaphylactic shock.
Author contributions
All authors contributed to the concept of study, acquisition
and analysis of data, drafting of the manuscript, and critical
revision of the manuscript for important intellectual content.
All authors had full access to the data, contributed to the
study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki. The patient provided verbal informed consent for
publication of this de-identified case report, including clinical
photos.
References
1. Dang TC, Nguyen TH, Do TD, et al. A human case of
subcutaneous dirofilariasis caused by Dirofilaria repens in
Vietnam: histologic and molecular confirmation. Parasitol
Res 2010;107:1003-7. Crossref
2. To KK, Wong SS, Poon RW, et al. A novel Dirofilaria species causing human and canine infections in Hong
Kong. J Clin Microbiol 2012;50:3534-41. Crossref
3. Eberhard ML. Zoonotic filariasis. In: Guerrant R, Walker D,
Weller P, editors. Tropical Infectious Diseases: Principles,
Pathogens, and Practice. 3rd ed. New York: Elsevier; 2011:
750-8. Crossref
4. Hou H, Shen G, Wu W, et al. Prevalence of Dirofilaria immitis infection in dogs from Dandong, China. Vet
Parasitol 2011;183:189-93. Crossref
5. Wang J, Zhu X, Ying Z, et al. Prevalence of Dirofilaria immitis infections in dogs and cats in Hainan Island/Province and three other coastal cities of China based on
antigen testing and PCR. J Parasitol 2019;105:199-202. Crossref
6. Wong SS, Teng JL, Poon RW, et al. Comparative evaluation
of a point-of-care immunochromatographic test SNAP
4DX with molecular detection tests for vector-borne
canine pathogens in Hong Kong. Vector Borne Zoonotic
Dis 2011;11:1269-77. Crossref
7. Kitoh K, Katoh H, Kitagawa H, Nagase M, Sasaki N,
Sasaki Y. Role of histamine in heartworm extract-induced
shock in dogs. Am J Vet Res 2001;62:770-4. Crossref
8. Khanna P, Garg R, Pawar D. Intraoperative anaphylaxis
caused by a hepatic hydatid cyst. Singapore Med J
2011;52:e18-9.
9. Minciullo PL, Cascio A, Gangemi S. Association between
urticaria and nematode infections. Allergy Asthma Proc
2018;39:86-95. Crossref
10. Simons FE, Ardusso LR, Bilò MB, et al. World allergy
organization guidelines for the assessment and
management of anaphylaxis. World Allergy Organ J
2011;4:13-37. Crossref

