© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Haemophagocytic lymphohistiocytosis
secondary to dengue fever: a case report
SW Cheo, MRCP (UK)1; WNFA Abdul Rashid, MB, BS (UM)1; CV Ho, MPath (UPM)2; Rosdina Z Ahmad Akhbar, MMed (UiTM)1; QJ Low, MRCP (UK)3; Giri S Rajahram, FRCP (UK)4
1 Department of Internal Medicine, Hospital Lahad Datu, Sabah, Malaysia
2 Department of Pathology, Hospital Queen Elizabeth, Sabah, Malaysia
3 Department of Internal Medicine, Hospital Sultanah Nora Ismail, Johor, Malaysia
4 Department of Internal Medicine, Hospital Queen Elizabeth, Sabah, Malaysia
Corresponding author: Dr SW Cheo (cheosengwee@gmail.com)
Case presentation
A 30-year-old man with underlying microcytic
hypochromic anaemia presented to a local health
clinic with a 3-day history of fever and 1-day
history of arthralgia, myalgia, abdominal pain, and
vomiting. On presentation, he was hypotensive
at 84/50 mm Hg and tachycardic with pulse rate
118 beats per minute. He responded well to fluid
resuscitation and was referred to hospital. Upon
arrival, he was alert with normal Glasgow Coma
Scale score, blood pressure 126/82 mm Hg, pulse
rate 126 beats per minute and temperature 37.7°C.
Examination revealed jaundiced, cold peripheries,
poor pulse volume, and a capillary refill time >2 s.
Respiratory examination showed crepitations over
the lung bases bilaterally. Systemic examination was
otherwise unremarkable.
His initial full blood count revealed
haemoglobin of 9.8 g/dL, white cell count
6.37 × 109/L, and platelet count of 30 × 109/L. He had
a deranged renal profile with sodium 134 mmol/L,
potassium 5.2 mmol/L, urea 11.6 mmol/L, and creatinine 211 μmol/L. He was also acidotic with
pH 7.39 and bicarbonate 14.3 mmol/L. Liver function
biochemistry showed elevated transaminases,
alanine aminotransferase 1114 U/L, aspartate
aminotransferase 1816 U/L, and lactate 5.26 mmol/L
(Table). He tested positive for dengue NS-1 and
negative for dengue immunoglobulin M and
immunoglobulin G. Blood smear for malaria parasite
and Leptospira immunoglobulin M was negative. He
was diagnosed with severe dengue, decompensated
shock, and multiorgan failure.
He was admitted to the intensive care unit
and treated with fluid resuscitation and blood
transfusion. Despite prompt initial resuscitation,
his clinical response was poor with further
deterioration in organ function. Elective intubation
and urgent haemodialysis were performed but
he collapsed prior to completion of the dialysis
session. Haemophagocytic lymphohistiocytosis
(HLH) was suspected in view of the multiorgan
failure and rapid clinical deterioration. Workup
revealed hypertriglyceridaemia of 8.7 mmol/L and hyperferritinaemia of >40 000 mg/L. Abdominal
ultrasound showed splenomegaly. There was no
family history of HLH. Unfortunately, he deteriorated
further and progressed to disseminated intravascular
coagulation, succumbing on day 3 of admission due
to multiorgan failure. Histopathological examination
of post-mortem bone marrow trephine biopsy
confirmed the diagnosis of HLH (Fig). His dengue
polymerase chain reaction test was later reported to
be positive for DEN3 infection.
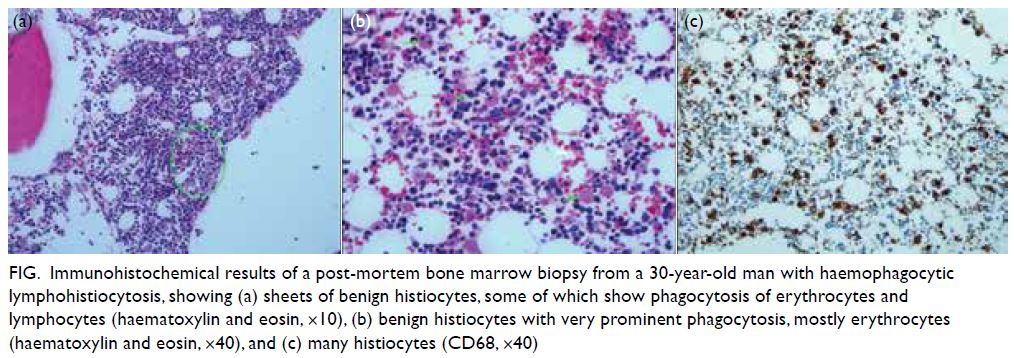
Figure. Immunohistochemical results of a post-mortem bone marrow biopsy from a 30-year-old man with haemophagocytic lymphohistiocytosis, showing (a) sheets of benign histiocytes, some of which show phagocytosis of erythrocytes and lymphocytes (haematoxylin and eosin, ×10), (b) benign histiocytes with very prominent phagocytosis, mostly erythrocytes (haematoxylin and eosin, ×40), and (c) many histiocytes (CD68, ×40)
Discussion
Haemophagocytic lymphohistiocytosis is a rare but
potentially life-threatening condition caused by
overactive immune activation. It was first described
by Farquhar and Claireaux in 1952.1 Broadly, it
can be divided into primary and secondary HLH.
Primary HLH typically manifests in children with
genetic abnormalities of natural killer cells and
T cells. Secondary HLH is often associated with
various infections that may be viral, bacterial,
fungal or parasitic, and connective tissue disorders
or malignancies, particularly T cell lymphoma.2
Dengue fever is a viral infection that can trigger
secondary HLH. In recent years, more reports
of dengue-associated HLH have emerged. It is
important for clinicians to recognise this entity
because it is associated with considerable mortality
and morbidity.
Dengue fever is an arboviral disease caused
by dengue virus, a virus of the Flaviviridae group.
Worldwide, it is endemic in more than 100 countries.
The World Health Organisation has estimated
there to be 390 million dengue infections annually
with 96 million manifesting clinically. It usually
presents with fever, myalgia, arthralgia, eye pain,
and headache. Around 5% of patients will progress
to severe dengue, characterised by plasma leakage,
hypovolaemic shock, haemorrhage, organ failure,
and encephalopathy.3 Some patients with severe
dengue will develop HLH.
Dengue is an uncommon cause of HLH,
but it should be suspected in patients with
unexplained systemic inflammatory response
syndrome such as prolonged fever, cytopenias,
malaise, and hepatosplenomegaly. Ongoing fever
after 8 days of illness should alert clinicians to
the possibility of HLH.4 Laboratory findings will
show cytopenia, raised ferritin, triglyceride, liver
impairment, hypofibrinogenaemia, and raised
lactate dehydrogenase. The diagnosis of HLH can be
established in the presence of a molecular diagnosis
consistent with HLH or the presence of five out
of eight criteria: fever >38.5°C; splenomegaly;
peripheral blood cytopenias; hypertriglyceridaemia;
hypofibrinogenaemia; haemophagocytosis in bone
marrow, spleen or liver; hyperferritinaemia
(>500 ng/mL); and increased CD25/interleukin-2
receptor or reduced natural killer cell function.4
The hallmark of diagnosis is observation of
haemophagocytosis in the tissue. Molecular
diagnosis consistent with HLH includes pathologic
mutations of PRF1, UNC13D, Munc18-2, Rab27a,
STX11, SH2D1A, or BIRC4.
Pathophysiologically, viral infection of T
cells leads to overproduction of cytokines such
as tumour necrosis factor alpha and interferon
gamma and can lead to uncontrolled histiocytic
activity. The consequent cytokine storm can lead
to organ dysfunction and death. To date, only three
serotypes of dengue virus (DEN1, DEN3 and DEN4)
are known to cause HLH. Our patient fulfilled
six of the HLH diagnostic criteria: having fever,
splenomegaly, cytopenias, hypertriglyceridaemia,
hyperferritenaemia, and haemophagocytosis in the
bone marrow. He also exhibited raised bilirubin,
liver enzymes and raised lactate dehydrogenase,
and developed acute renal failure that required
haemodialysis. Unfortunately, he became
haemodynamically unstable during dialysis and
eventually succumbed to his illness. Fibrinogen and
CD25 levels were not measured as the tests were not
available in our centre.
In the absence of treatment, dengueassociated
HLH carries a high mortality.5 Essentially,
it is important to suspect and diagnose the clinical
syndrome early so that appropriate treatment
can be given. In general, management of dengue-associated
HLH includes standard fluid protocols
and HLH-directed therapy. Dexamethasone and
etoposide can be given as HLH-directed therapy
to suppress the overactive immune response. The
exact mechanism of etoposide in hyperinflammation
is not well understood but it has been shown to
alleviate symptoms of all murine HLH.5 As well
as corticosteroid and etoposide, intravenous
immunoglobulin and antithymocyte globulin have
also been tried. However, clinicians should remain
vigilant when administering HLH-directed therapy
in the setting of concomitant sepsis.
Conclusion
Dengue-associated HLH is an important and unique
entity. We believe that it is very much underreported
due to failed recognition of the entity. The hallmark
of this disease is an overactive immune response and
presence of haemophagocytosis. Dengue-associated
HLH can be diagnosed by HLH criteria and HLH-directed
therapy initiated.
Author contributions
Concept or design: All authors.
Acquisition of data: SW Cheo.
Analysis or interpretation of data: SW Cheo, CV Ho, RZ Ahmad Akhbar, QJ Low.
Drafting of the manuscript: SW Cheo, WNFA Abdul Rashid, QJ Low.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: SW Cheo.
Analysis or interpretation of data: SW Cheo, CV Ho, RZ Ahmad Akhbar, QJ Low.
Drafting of the manuscript: SW Cheo, WNFA Abdul Rashid, QJ Low.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Acknowledgement
The authors would like to thank Tan Sri Dato' Seri Dr Noor Hisham Abdullah, the Director General of Health Malaysia
for his permission to publish this article.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration
of Helsinki. The patient provided informed consent for all
treatments and procedures, and the patient’s brother provided
consent for publication.
References
1. Farquhar JW, Claireaux AE. Familial haemophagocytic
reticulosis. Arch Dis Child 1952;27:519-25. Crossref
2. Ray U, Dutta S, Mondal S, Bandyopadhyay S. Severe dengue
due to secondary hemophagocytic lymphohistiocytosis: a
case study. IDCases 2017;8:50-3. Crossref
3. Ellis EM, Sharp TM, Pérez-Padilla J, et al. Incidence and risk
factors for developing dengue-associated hemophagocytic
lymphohistiocytosis in Puerto Rico, 2008-2013. PLoS Negl
Trop Dis 2016;10:e0004939. Crossref
4. Koshy M, Mishra AK, Agrawal B, Kurup AR, Hansdak SG.
Dengue fever complicated by hemophagocytosis. Oxf Med
Case Reports 2016;2016:121-4. Crossref
5. Kan FK, Tan CC, Greenwood TVB, et al. Dengue infection
complicated by hemophagocytic lymphohistiocytosis:
experiences from 180 patients with severe dengue. Clin
Infect Dis 2020;70:2247-55. Crossref


