© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE CME
Initial intravenous fluid prescription in general
paediatric in-patients aged >28 days and <18 years: consensus statements
Lettie CK Leung, FRCP, FHKCPaed1; LY So, FHKCPaed, FRCPCH2; YK Ng, FHKCPaed3; Winnie KY Chan, FHKCPaed, FRCPCH4; WK Chiu, FHKCPaed, FRCPCH5; CM Chow, FHKCPaed, FHKAM (Paediatrics)6; SY Chan, RN, MSc (HSM)4; KC Chan, FHKCPaed, FRCPCH7; for the IVF Working Group
1 Department of Paediatrics, Kwong Wah Hospital, Hong Kong
2 Department of Paediatrics and Adolescent Medicine, Pamela Youde Nethersole Eastern Hospital, Hong Kong
3 Department of Paediatrics and Adolescent Medicine, Queen Mary Hospital. Hong Kong
4 Department of Paediatrics, Queen Elizabeth Hospital, Hong Kong
5 Department of Paediatrics and Adolescent Medicine, United Christian Hospital, Hong Kong
6 Department of Paediatrics, Prince of Wales Hospital, Hong Kong
7 Department of Paediatrics and Adolescent Medicine, Alice Ho Miu Ling Nethersole Hospital, Hong Kong
Corresponding author: Dr Lettie CK Leung (leungckl@ha.org.hk)
Abstract
Intravenous fluid (IVF) prescription has often
been an ‘assumed’ skill in hospital-based paediatric
practice, with little evidence-based guidance.
Traditionally prescribed hypotonic fluids were
responsible for many iatrogenic, hyponatraemia-related
morbidity and mortality. Robust evidence is
available to support recent guidelines that isotonic
fluids are the most appropriate maintenance IVF for
most hospitalised children. However, many other
aspects of IVF prescription still lack evidence. Thus,
an IVF Working Group was formed in 2016 under
the Hospital Authority Paediatric Coordinating
Committee to review IVF guidelines for local
application, with the aim to provide guidance for
initial IVF prescription and subsequent monitoring
of paediatric in-patients in Hong Kong. Published
randomised controlled trials, IVF guidelines, and
practices of reputable children’s hospitals up to
December 2019 were reviewed. Local survey findings
and practical realities were considered. Extracted
evidence and draft recommendations were presented
to the group, using a consensus approach in areas
where evidence was unavailable. After further input
from designated reviewers, an IVF clinical pathway
was finalised in November 2019 and endorsed by
the Paediatric Clinical Coordinating Committee.
This article represents an explanatory discussion of
the pathway, with consensus statements established
by Working Group members at the final meeting in
June 2020. The consensus statements emphasise that IVF should be prescribed with the same care and
consideration as medications, based on each patient’s
pathophysiology. Evidence is presented regarding
the use of isotonic maintenance fluid, comparing
0.9% sodium chloride with balanced solutions.
These eight statements provide localised guidance
for paediatricians in initial IVF prescription but do
not replace clinical judgement.
Introduction
The prescription of intravenous fluid (IVF) is
an essential management modality in hospital
paediatrics. The traditional practice of administering
hypotonic maintenance IVF (0.18-0.3% sodium
chloride [NaCl]) was based on the Holliday–Segar
formula published in 19571; being calculated from
estimated fluid and electrolyte requirements based
on milk intake in healthy children. Holliday–Segar
formula for maintenance fluid in 24 hours is calculated
as below: (1) body weight (BW) of ≤10 kg: 100 mL/kg;
(2) BW of 11-20 kg: 1000 mL + 50 mL/kg for each kg
over 10; (3) BW of >20 kg: 1500 mL + 20 mL/kg for
each kg over 20. However, since the 1990s, more than 100 cases of hyponatremia-related iatrogenic death
or permanent neurologic impairment have been
reported; nearly all studies have shown that hospital-acquired
hyponatremia is related to hypotonic fluid
administration.2 This is related to the high incidence
of non-osmotic stimuli of antidiuretic hormone
(SIADH) in sick children, which leads to an impaired
ability to excrete free water.
Since the 1990s, there have been many
randomised controlled trials (RCTs) and meta-analyses
comparing hypotonic and isotonic fluids
in children initially in postoperative and paediatric
intensive care unit settings, but recently also in
general paediatric settings. Based on the available high-quality evidence, the 2015 NICE guideline3 and
the American Academy of Pediatrics maintenance
fluid guidelines4 have strongly recommended the
use of isotonic maintenance IVF for most paediatric
patients aged >28 days.
In Hong Kong, there are no local guidelines
regarding IVF prescription in children. A 2016
informal survey of 11 acute paediatric units within
the Hospital Authority showed that dextrose-containing
0.3% NaCl and 0.45% NaCl were the
main maintenance fluids used; no units routinely
used isotonic fluids. Our recent survey5 of more
than 60 000 hospital admissions of children aged
1 month to 18 years in the year 2015 also showed
variations among hospitals in terms of IVF practices.
Hyponatraemia commonly occurred in 8.8% of
admissions. In total, 110 patients exhibited true, non-dilutional
severe hyponatraemia (<127 mmol/L);
this was hospital-acquired in 22 patients
(presumably related to hypotonic IVF) and some of
them exhibited neurological complications. In this
context, the current practice statement is intended
to guide all clinicians who prescribe IVF in children,
encouraging methodological prescription practices
to minimise fluid and electrolyte morbidities.
Basic intravenous fluid concepts
Osmolality versus tonicity
Osmolality is the concentration of a solution expressed as the number of solute particles per
kilogram of solution (plasma). Tonicity is a measure
of the effective osmolality between two fluid
compartments separated by a semi-permeable
membrane (eg, a cell membrane). For our purposes,
tonicity refers to the sodium concentration of the
fluid. Dextrose does not affect tonicity because it is
rapidly metabolised in the blood stream.
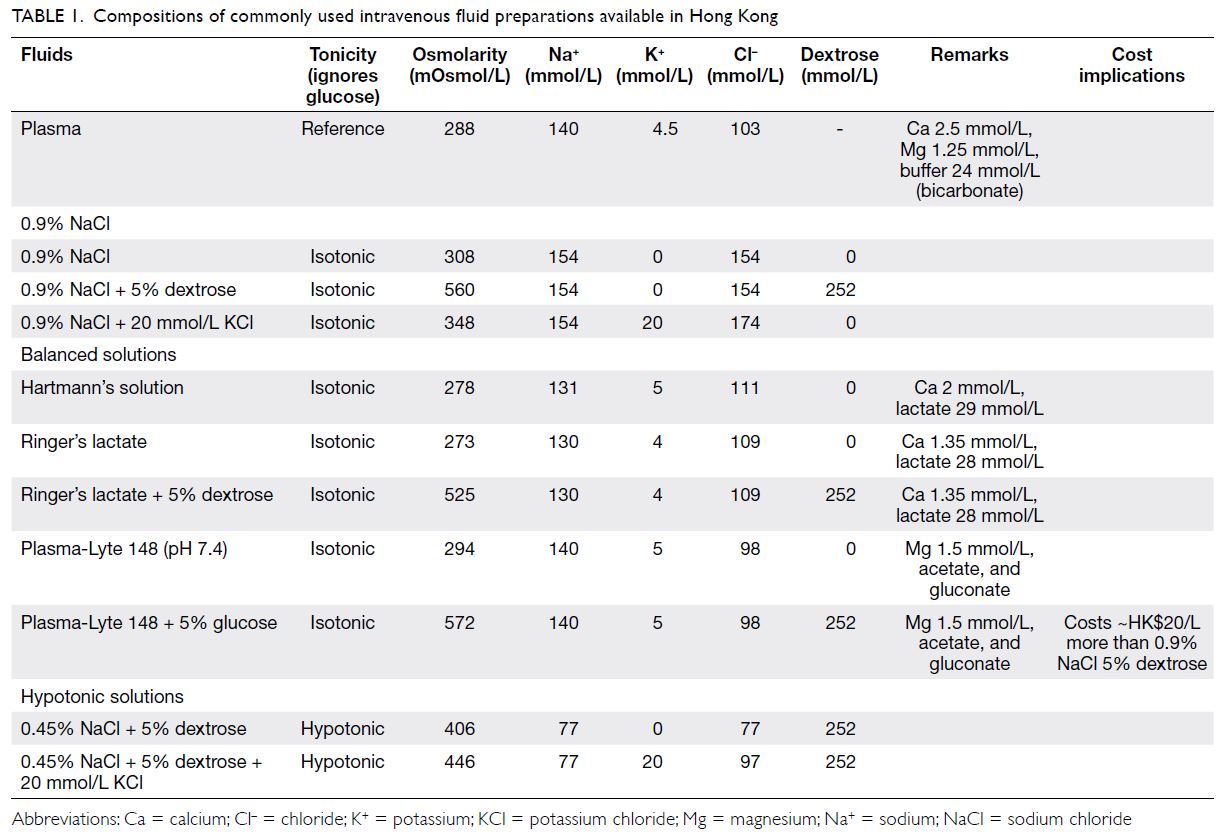
Normal saline versus balanced solution
The compositions of available IVF preparations in Hong Kong are detailed in Table 1. Normal saline
(0.9% NaCl) may not be as “normal” as its name
suggests. Compared with plasma, 0.9% NaCl has
higher sodium (Na 154 vs 140 mmol/L) and chloride
(Cl 154 vs 103 mmol/L) concentrations; moreover,
large volumes of 0.9% NaCl lead to hyperchloraemic
acidosis in adults and children.6
Balanced solutions are solutions with more
physiologically appropriate electrolyte compositions
(Table 1). They contain buffers with mild alkalinising
effects. For example, Hartman’s and Ringer’s
lactate solutions contain sodium lactate, which is
metabolised into bicarbonate by the liver. Plasma-Lyte 148 contains acetate, which is similarly
metabolised (within 15 minutes) by the liver and
skeletal muscle. Acetate metabolism has several
advantages: it is not entirely dependent on hepatic function, it is preserved in severe shock, and it will not
disrupt reported serum lactate levels. Furthermore,
Hartman’s and Ringer’s lactate solutions contain
calcium, which may contribute to extravasation and
cause incompatibility with blood or drugs such as
cefotaxime. In contrast, Plasma-Lyte 148 contains
magnesium, for which limited drug compatibility
information is available.
There is increasing evidence that 0.9% NaCl is
associated with increased rates of mortality, acute
kidney injury, metabolic acidosis, and coagulopathy
in critically ill adults7 8 and acute kidney injury in
non–critically ill adults,9 compared with balanced
solutions. These effects have been attributed to its
supraphysiologic chloride concentration, which
causes renal vasoconstriction.6 10 In children with
septic shock, hyperchloraemia is also associated
with acute kidney injury and mortality11 12 13; this
implication will be discussed later.
Methods
After publication of the NICE IVF guideline, an IVF
Working Group (ie, the IVFWG) was formed in June
2016 under the Evidence-based Practice Working
Group of the Hospital Authority Paediatric Clinical
Coordinating Committee to form recommendations
regarding maintenance fluid prescription. A
retrospective survey of hyponatraemia with a
focus on IVF practices was commissioned and
published5; lessons from the survey were taken into
consideration. Additionally, a literature search was performed using the keywords (“intravenous fluid”
OR “isotonic” OR “hypotonic” OR “maintenance
fluid”) AND (“child”) in MEDLINE and EMBASE
databases; results from January 2000 to December
2019 were collected. All RCTs and meta-analyses
regarding maintenance IVF were reviewed. For our
review (ie, maintenance fluids in general paediatric
settings), we only included paediatric RCTs where
medical patients constituted more than 50% of the
study population. We excluded studies in which
surgical or intensive care patients comprised the
majority of patients, as well as studies in which
IVF served both rehydration and maintenance
purposes. The certainty of evidence and strength
of each recommendation were determined in
accordance with GRADE methodology (https://www.gradeworkinggroup.org/"). Extracted evidence
was presented to the IVFWG. In areas for which
the evidence level was high, clear recommendations
were made. In areas for which trial data were
lacking, the preferred treatment was determined by
a consensus approach based on the knowledge and clinical experience of the IVFWG members.
The first draft recommendations were presented
to IVFWG members in February 2019. After
publication of the American Academy of Pediatrics
guideline4 in December 2018, IVF guidelines were
amended by many reputable international children’s
hospitals. These amended guidelines were also
used as reference information; their applicability to
patients in Hong Kong were further discussed within
the group. The consensus document was submitted
to a panel of five external reviewers chosen by the
Evidence-based Practice Working Group to widen
input from all subspecialty sectors. In accordance
with the reviewers’ comments, a clinical pathway
was finalised in November 2019 (Fig) and endorsed
by the Paediatric Clinical Coordinating Committee.
This article represents an explanatory discussion of
the pathway, with consensus statements established
by IVFWG members at the final meeting in June
2020. In this article, each statement includes an
indication of whether it was established on the basis
of evidence or IVFWG member consensus.
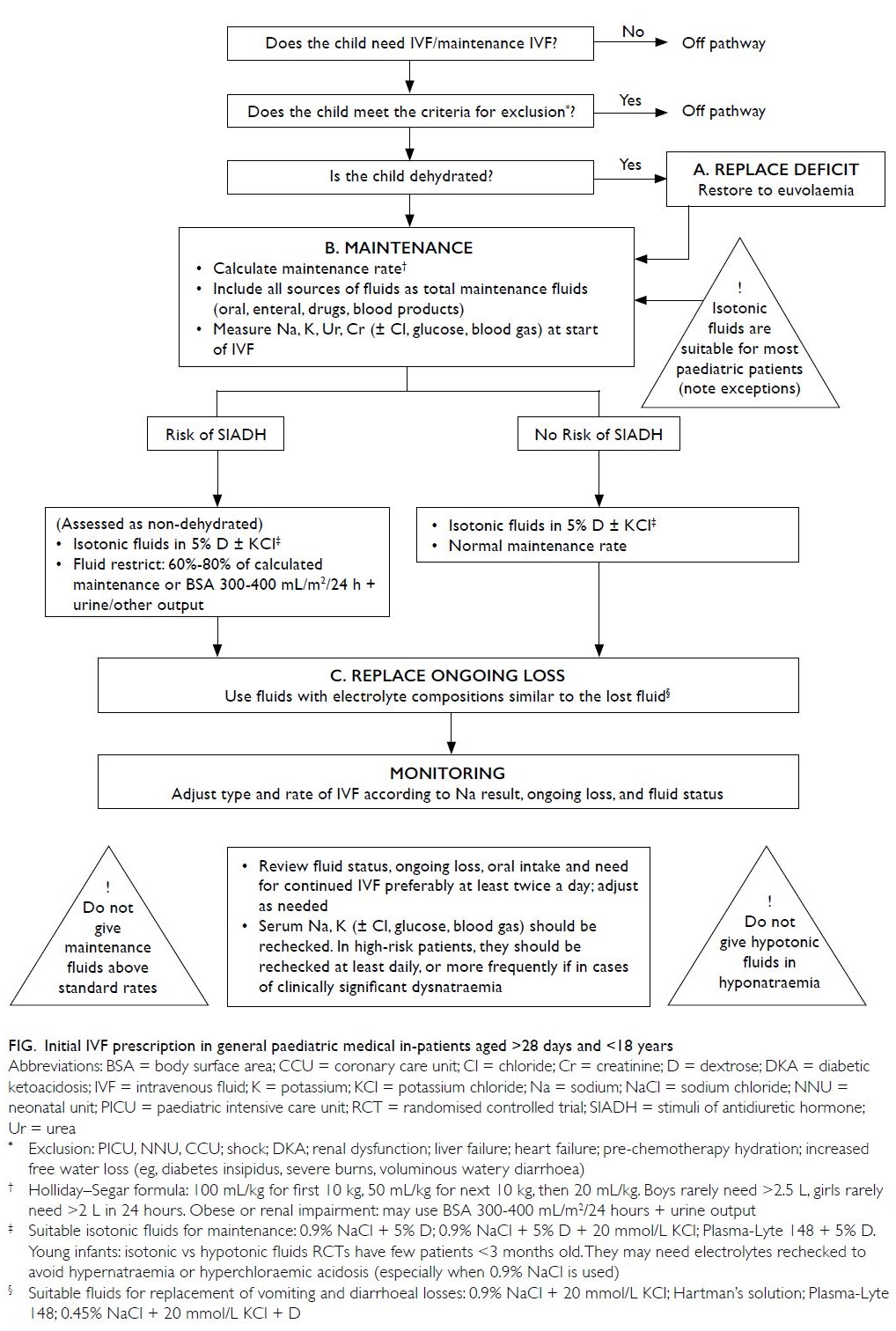
Figure. Initial IVF prescription in general paediatric medical in-patients aged >28 days and <18 years
Scope of consensus statements
These consensus statements aim to facilitate initial
IVF prescription (ie, prescription principles, with
a focus on maintenance fluids) and subsequent
monitoring for general paediatric in-patients. They
are not meant to replace clinical judgement, nor do
they represent a comprehensive discourse regarding
fluid resuscitation, specific conditions, or treatment
of dysnatraemia.
Target population
The target population for these statements comprises
children aged >28 days to <18 years who have been
admitted to a general paediatric ward for medical
conditions. Based on the exclusion criteria of the
majority of prospective studies of maintenance
fluids, Table 2 lists patients excluded from guidance
in these statements. Their IVF needs should be
individually assessed (see Statement 2).
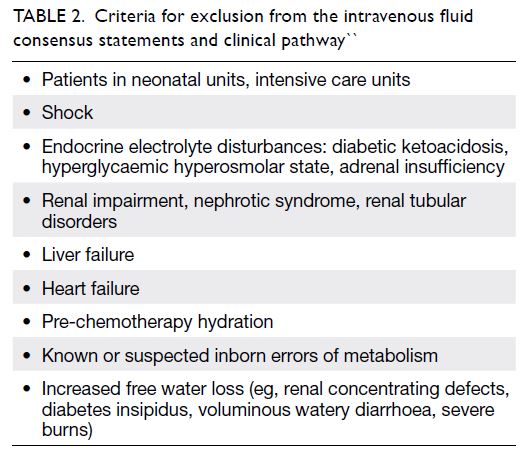
Table 2. Criteria for exclusion from the intravenous fluid consensus statements and clinical pathway
Consensus statements
Statement 1. Intravenous fluid should be
administered only when the enteral route is
considered inappropriate or inadequate; IVF
should be discontinued once enteral route
can be substituted. (Consensus)
Enteral fluid administration is always safer and
preferable because the child can autoregulate
the amount of ingested fluid. The indication
and continued need for IVF should be reviewed
regularly; IVF should be stopped when enteral
intake is adequate. If the patient is unable to increase
enteral intake and has been receiving IVF for 5 days,
parenteral nutrition should be considered.
Statement 2. Intravenous fluid should
be prescribed with the same care and
consideration as used for medication.
Individual clinical situations must be
assessed, with specific attention to the
patient’s volume status, pathophysiological
and biochemical state. (Consensus)
No single solution can provide maintenance water and electrolyte needs for all children because needs
vary among individuals and disease states. It is
crucial to understand the pathophysiological state of
a particular patient (Table 314). Before prescribing,
clinicians should ask: Why am I prescribing IVF?
What disease state, abnormal volume status, and
water or electrolyte imbalance am I starting with?
Based on these considerations, which type and rate
of fluid should I choose? How will I monitor the
effects and side-effects of my treatment, with the
awareness that requirements may change as the
patient’s condition evolves?

Table 3. Understanding the patient’s pathophysiology: conditions requiring special considerations when intravenous fluids are prescribed (modified from Moritz et al14)
Statement 3. When IVF is prescribed, the
three components of prescription (deficit
replacement, maintenance, and management
of ongoing loss) should be considered
separately. (Consensus)
The goal of IVF is maintenance of fluid and electrolyte
homeostasis. Hypovolaemia is a physiological
stimulus for antidiuretic hormone release; therefore,
deficits should be replaced to achieve euvolaemia.
Patients then require IVF to maintain their
ideal volume status, with continued clinical and
biochemical monitoring of ongoing loss. These three
components require different considerations of fluid
types and rates. Fluids intended to replace deficits
or ongoing losses may be co-administered with
maintenance fluids for easier adjustments.
A clinical pathway depicting initial IVF
prescription for general paediatric in-patients is
illustrated in the Figure.
Statement 4. Replacement of fluid deficit
should usually be with non-glucose-containing
isotonic fluids at the appropriate
rate. (Consensus)
Deficit replacement fluids restore hydration by
replacing fluids already lost. Volume deficits are
isotonic deficits; therefore, they should normally
be replaced with isotonic fluids at the appropriate
rate for the patient’s particular pathophysiological
state. For example, 10 to 20 mL/kg/hour for 2 to
4 hours may be appropriate for a normonatraemic
dehydrated child with gastroenteritis without
shock.15 16 The rate should be slower in patients with
pre-existing cardiac or renal disease; the deficit
replacement volume should be administered over a
longer period (eg, 48 hours) in patients with diabetic
ketoacidosis or hypernatraemic dehydration.
4.1 Regarding evidence for fast (20-60 mL/kg in 1-2 hours) versus slow (in 2-4 hours) rates
of fluid replacement in patients with acute
gastroenteritis, available RCTs have shown
heterogeneous results and have generally been
conducted in resource-limited settings.17 Fast
rates of rehydration have not demonstrated
clearly superior results. Considering recent
concerns regarding aggressive fluid expansion,
more research is warranted before guidelines
can be established.
4.2 Notably, 0.9% NaCl has been the traditional fluid
of choice for both volume resuscitation and
deficit replacement. As previously mentioned,
there are concerns that high volumes of 0.9%
NaCl cause hyperchloraemia-related adverse
effects in critically ill adults and children.6 Some
paediatric anaesthetist guidelines18 19 favour
administration of balanced fluids over 0.9% NaCl for resuscitation/replacement. However,
there is no evidence thus far from small studies
in non–critically ill children that balanced
solutions are superior.20 21 It may be prudent
for clinicians to monitor for hyperchloraemia
and consider the use of more physiologically
appropriate solutions in sick children.22
Statement 5. Initial IVF and maintenance
IVF types: most children aged >28 days to
<18 years should receive isotonic solutions
with appropriate potassium chloride and
dextrose as maintenance IVF. (High-quality
evidence, strong recommendation)
Intravenous fluid administration is intended to
meet anticipated water and electrolyte needs
from insensible losses and urinary output. When prescribing initial IVF, clinicians should consider
that most hospitalised children are at risk of osmotic
(appropriate) and non-osmotic (inappropriate)
antidiuretic hormone secretion (Table 3), causing an
inability to excrete free water through dilute urine.
This puts the child at risk of positive water balance
and hyponatraemia when hypotonic fluids are
administered.
5.1 Earlier RCTs regarding fluid tonicity were
mainly in surgical and paediatric intensive care
unit patients. Our literature search revealed
high-quality evidence from 10 RCTs involving
general paediatric in-patients, indicating that
isotonic fluids significantly reduce the risk of
hyponatraemia compared with hypotonic fluids
(including 0.45% NaCl). Table 423 24 25 26 27 28 29 30 31 32 lists the
characteristics and GRADE evidence levels of
these studies. Most RCTs used 0.9% NaCl with
20 mmol/L potassium chloride (KCl) in the
isotonic arm, whereas the PIMS trial,25 which
included >690 children used Plasma-Lyte 148.
Eight of the 10 studies included 0.45% NaCl
in the hypotonic fluid arm. Study appraisal of
these RCTs (total 1945 patients) showed that
eight of the 10 were methodologically sound
(GRADE evidence level ≥3). Hence, high-quality
evidence indicates that hypotonic fluids
(including 0.45% NaCl) carry a significantly
greater risk of hospital-acquired hyponatraemia
(relative risk=3.7-6.5), as well as a risk of failed
sodium status improvement in patients with
baseline hyponatraemia.
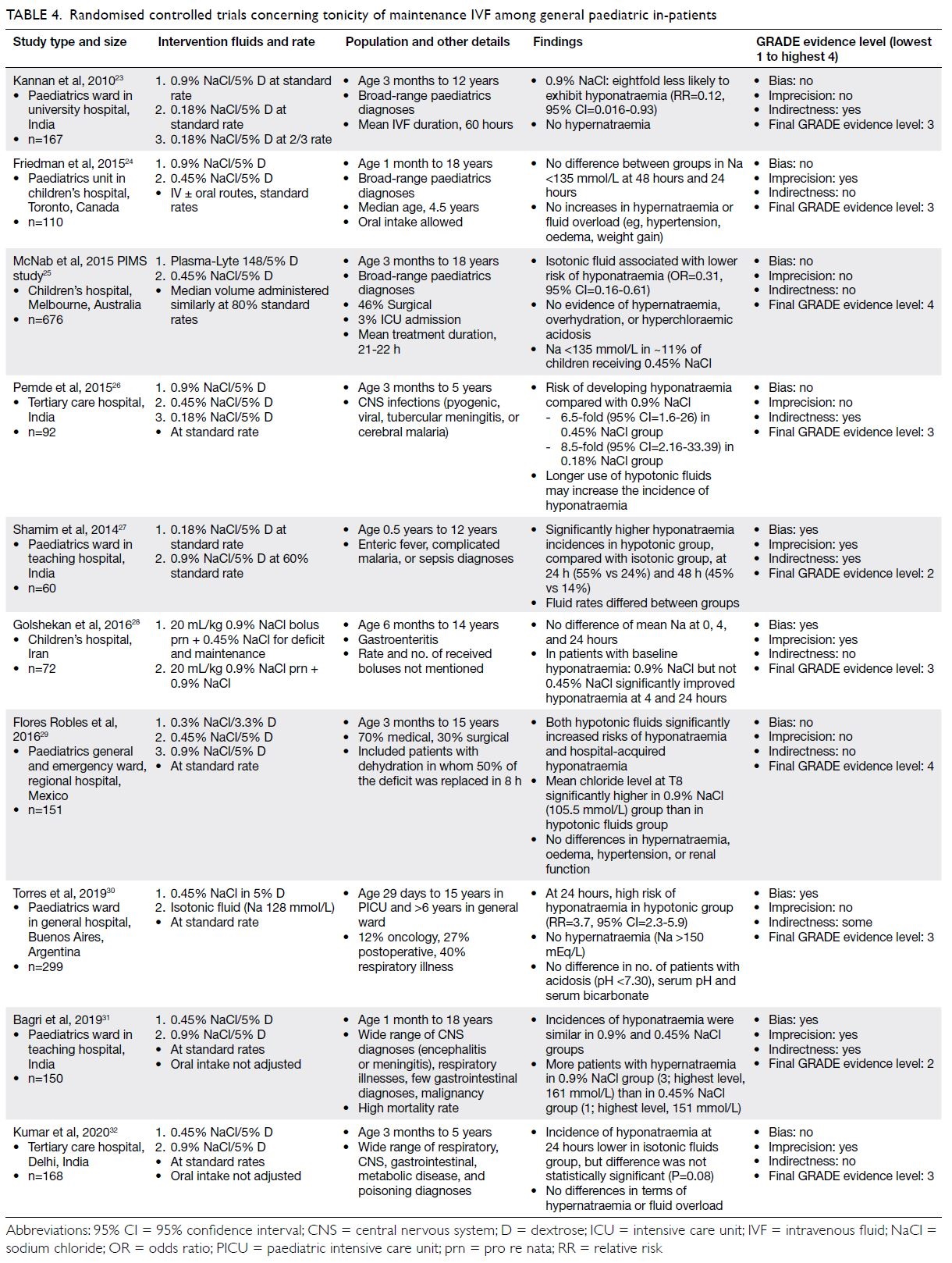
Table 4. Randomised controlled trials concerning tonicity of maintenance IVF among general paediatric in-patients
5.2 Regarding potential harm, there is no evidence
from these studies that isotonic maintenance
fluids increase the risk of hypernatraemia.
However, other side-effects (eg, fluid overload,
hypertension, and hyperchloraemic acidosis)
have not been sufficiently evaluated. This
highlights the importance of continuous
monitoring.
5.3 Three isotonic fluids containing 5% dextrose
(D5) are available in Hong Kong: 0.9% NaCl
D5, Ringer’s lactate D5 balanced solution,
and Plasma-Lyte 148 D5 balanced solution
(compositions listed in Table 1). Guidelines
adopting robust methodologies3 4 have not
indicated a preference for any particular isotonic
fluid composition; however, Children’s Hospital
Colorado guidelines indicate a preference for
balanced solutions (rather than 0.9% NaCl) as
maintenance fluid for all age ranges, citing the
need to monitor for hyperchloraemic acidosis
with ‘unbalanced’ 0.9% NaCl.33 However,
because there is a lack of direct comparative
studies, this recommendation is more opinion-than
evidence-based.
5.4 A note of caution is needed regarding the selection of isotonic fluids in young infants
because most RCTs recruited infants from
age 3 months; all RCTs contained few young
infant patients. Because of their immature
kidneys, young infants may require electrolyte
monitoring to ensure hypernatraemia and/or
hyperchloraemic acidosis do not occur, especially
when 0.9% NaCl is used. Thus, we suggest using
dextrose-containing balanced solutions with
lower NaCl content as maintenance IVF for
patients aged 1 to 3 months.
5.5 When maintenance IVF treatment is considered
for older infants, the widely available 0.9% NaCl
D5 is a suitable choice, especially if the treatment
is supplementary or will be administered for
short-term use. However, because of its high
chloride content, clinicians should consider the
potential risk of hyperchloraemic acidosis when
0.9% NaCl D5 is used in large volumes or for
long durations, particularly in sick patients.
5.6 The slightly more expensive (HK$20/L more)
Plasma-Lyte 148 D5 balanced solution is also
a suitable isotonic solution for general use,
especially in sick patients or patients exhibiting
shock because of its lower chloride content.
The mild alkalinising effect of this solution
may benefit patients with acidaemia, although
caution is needed when the solution is used
in patients with hypocalcaemia or metabolic
alkalosis. Plasma-Lyte 148 D5 balanced
solution contains potassium at physiologically
appropriate concentrations which can provide
maintenance needs, but it should not be used in
patients with hyperkalaemia.34
5.7 In the uncommon situations involving
free water deficit, excessive non-renal or
renal free water, or hypotonic fluid loss
(Table 3), hypotonic fluids may be needed.
These situations are usually associated with
hypernatraemia, which should be corrected
slowly (at a concentration <10 mmol/L/24 hours).
Paired serum and urinary osmolarity and
electrolyte monitoring are helpful in these
situations.
5.8 There is no evidence-based recommendation
regarding the addition of KCl, although
many guidelines suggest the addition of 10
to 20 mmol/L KCl to maintenance IVF after
confirmation of normal serum potassium and
creatinine levels, as well as confirmation that
there is no risk of renal impairment. Potassium
supplementation is important when there is
a delay in reinitiation of oral intake. Balanced
solutions generally do not require additional
potassium supplementation,33 though their
physiological KCl concentration is inadequate
to treat hypokalaemia.
Statement 6. Calculations of maintenance
IVF rate should include all oral, enteral,
drug, and blood products, normally using
the Holliday–Segar formula. Patients at
risk of SIADH may require fluid restriction.
(Consensus)
Traditionally, daily IVF volumes should be calculated
using the Holliday–Segar formula. However,
evidence regarding appropriate fluid volumes in
hospitalised children is lacking. McNab et al35
examined four (mostly surgical) RCTs which
included restricted rates in their intervention arms.
The limited evidence available36 37 showed that
0.45% NaCl at <70% maintenance rates did not
protect against hyponatraemia, suggesting that fluid
type is more important than fluid rate for prevention
of hyponatraemia.
The IVFWG has these consensus opinions,
pending more evidence:
6.1 When determining fluid volumes, volumes
calculated using the Holliday–Segar formula
should rarely exceed adult volumes (2 L/day for
girls and 2.5 L/day for boys) or 100 mL/hour.3
In patients for whom accurate calculation of
insensible water loss is important (eg, patients
with obesity, acute kidney injury, chronic kidney
disease, or cancer), body surface area may be
useful when calculating fluid requirements at
300 to 400 mL/m2/24 hours plus urine output3; or
in patients weighing >10 kg, fluid requirements
calculated as 1500 mL/m2/24 hours.38
6.2 In patients at risk of SIADH (Table 3), volumes may be restricted to 60% to 80% maintenance.
Patients with central nervous system conditions
(eg, meningitis, encephalitis, or major head
injury) may require fluid restriction to 50% for
management of cerebral oedema.
Statement 7. Ongoing fluid loss should
be replaced using fluids with comparable
electrolyte compositions. (Consensus)
7.1 Increased ongoing losses (eg, vomiting, diarrhoea, ostomy, and third space losses)
should be taken into account and replaced
with comparable fluids (Table 53 9 10 39 40 shows
electrolyte compositions of various body fluids).
For vomiting and non-choleric diarrhoea, both
0.45% NaCl or 0.9% NaCl solutions (with added
potassium) are recommended.
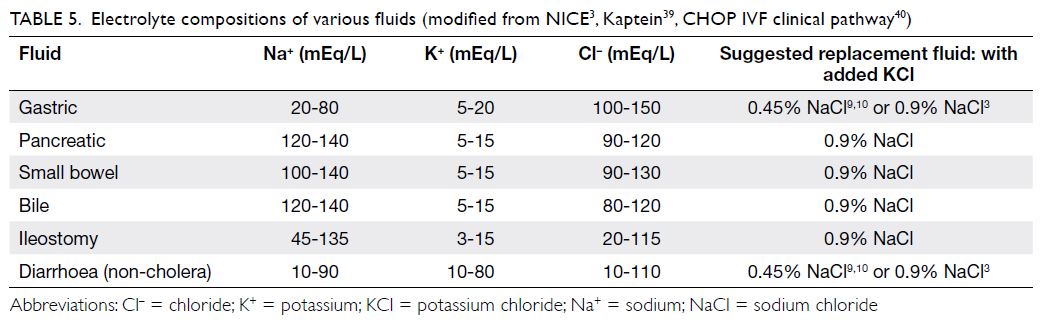
Table 5. Electrolyte compositions of various fluids (modified from NICE3, Kaptein39, CHOP IVF clinical pathway40)
7.2 Abnormal urine electrolyte losses can vary
widely; thus, monitoring of paired urine and
serum electrolyte levels, as well as creatinine
and osmolarity parameters, may be needed.
In some situations, even isotonic fluids may
be insufficient to prevent hyponatraemia (eg,
patients with central nervous system injury with
cerebral salt wasting or patients with SIADH in
whom urine osmolality is >500 mOsmol/kg);
these patients require measurement of urinary
electrolytes and osmolality.
Statement 8. All children receiving IVF
should undergo regular clinical and
biochemical monitoring to assess their
responses to therapy and changes in clinical
status. Monitoring frequencies should be
based on a risk assessment involving the
child’s age, clinical and volume statuses,
stability, IVF proportion, and presence of
biochemical abnormalities. (Consensus)
8.1 An infusion pump should be used for all children requiring maintenance IVF.
8.2 Children receiving IVF should have an accurate
weight recorded on admission or as soon as
clinically possible; daily weight should be
recorded as needed, specifically noting weight
fluctuations ± 3% in 24 hours. Daily fluid balance
(ie, input, output, and abnormal ongoing loss)
should be recorded.
8.3 Clinical assessment of fluid status (ie, body
weight, heart rate, capillary refill time,
hydration, and blood pressure), fluid balance,
oral fluid tolerance, and the continued need
for IVF should be reviewed often (preferably at
least twice daily).
8.4 The plasma electrolyte profile (sodium, potassium, urea, creatinine, chloride, and acid-base level) should be checked at the initiation
of IVF, then rechecked in accordance with
the risk level and proportion of maintenance
fluid supplied as IVF. In young infants, high-risk
patients, and patients receiving prolonged
IVF treatment, reassessments should be
performed at least daily or more frequently if
an electrolyte abnormality is present, or if the
patient is particularly unwell. The blood glucose
level should be checked if there is a risk of
hypoglycaemia (eg, in young infants). Paired
serum and urinary osmolarity and electrolyte
profiles may be useful to guide fluid prescription
in patients with electrolyte abnormalities.
Conclusion
While there is strong evidence that isotonic
solutions are the most appropriate maintenance
IVF for the vast majority of hospitalised children,
a reflexive approach to IVF prescription should be
avoided. Intravenous fluid should be prescribed
with the same care used for medications; with the
rate and type of fluid tailored to the individual’s
clinical and pathophysiological statuses. Regular
monitoring and reassessment with appropriate
fluid readjustment are critical considerations.
Many aspects of IVF treatment continue to exhibit
a lack of evidence, such as the selection of 0.9%
NaCl or balanced solution, as well as the fluid rate
and optimal potassium supplement composition.
When more evidence is available, these practice
statements with the accompanying algorithms
should be reviewed.
Author contributions
Concept or design: LCK Leung, KC Chan.
Acquisition of data: LCK Leung.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: LCK Leung.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: LCK Leung.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: LCK Leung.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors would like to thank reviewers of the Intravenous Fluid Clinical Pathway (Dr KW Hung, Dr YW Kwan, Dr SN
Wong, Dr SWC Wong, and Dr MM Yau) for their invaluable
comments.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
1. Holliday MA, Segar WE. The maintenance need for water
in parenteral fluid therapy. Pediatrics 1957;19:823-32.
2. Koczmara C, Wade AW, Skippen P, et al. Hospital-acquired
acute hyponatremia and reports of pediatric deaths.
Dynamics 2010;21:21-6.
3. National Institute for Health and Care Excellence.
Intravenous fluid therapy in children and young people
in hospital. NICE guideline [NG29]. December 2015.
Available from: https://www.nice.org.uk/guidance/ng29.
Accessed 2 Jul 2019.
4. Feld LG, Neuspiel DR, Foster BA, et al. Clinical practice
guideline: maintenance intravenous fluids in children.
Pediatrics 2018;142:e20183083. Crossref
5. Leung LC, Chan KC, Chan WK, et al. Hyponatraemia
in hospitalized children: a retrospective survey in acute
paediatric admissions in Hong Kong with focus on
intravenous fluid practices. HK J Paediatr (New Series)
2020;25:137-47.
6. Hoorn EJ. Intravenous fluids: balancing solutions. J
Nephrol 2017;30:485-92. Crossref
7. Semler MW, Self WH, Wanderer JP, et al. Balanced
crystalloids versus saline in critically ill adults. N Engl J
Med 2018;378:829-39. Crossref
8. Raghunathan K, Shaw A, Nathanson B, et al. Association
between the choice of IV crystalloid and in-hospital
mortality among critically ill adults with sepsis. Crit Care
Med 2014;42:1585-91. Crossref
9. Self WH, Semler MW, Wanderer JP, et al. Balanced
crystalloids vs saline in noncritically ill adults. N Engl J
Med 2018;378:819-28. Crossref
10. Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest 1983;71:726-35. Crossref
11. Stenson EK, Cvijanovich NZ, Anas N, et al. Hyperchloremia
is associated with complicated course and mortality in
pediatric patients with septic shock. Pediatr Crit Care Med
2018;19:155-60. Crossref
12. Barhight MF, Lusk J, Brinton J, et al. Hyperchloremia
is independently associated with mortality in critically
ill children who ultimately require continuous renal
replacement therapy. Pediatr Nephrol 2018;33:1079-85. Crossref
13. Emrath ET, Fortenberry JD, Travers C, McCracken CE,
Hebbar KB. Resuscitation with balanced fluids is associated
with improved survival in pediatric severe sepsis. Crit Care
Med 2017;45:1177-83. Crossref
14. Moritz ML, Ayus JC. Maintenance intravenous fluids in
acutely ill patients. N Engl J Med 2015;373:1350-60. Crossref
15. Ministry of Health, New South Wales Government. Infants
and children: Management of acute gastroenteritis. 4th
ed. Available from: https://www1.health.nsw.gov.au/pds/ActivePDSDocuments/GL2014_024.pdf. Accessed 2 Jul
2019.
16. Guarino A, Ashkenazi S, Gendrel D, et al. European
Society for Pediatric Gastroenterology, Hepatology, and
Nutrition/European Society for Pediatric Infectious
Diseases evidence-based guidelines for the management of
acute gastroenteritis in children in Europe: update 2014. J
Pediatr Gastroenterol Nutr 2014;59:132-52. Crossref
17. Iro MA, Sell T, Brown N, Maitland K. Rapid intravenous
rehydration of children with acute gastroenteritis and
dehydration: a systematic review and meta-analysis. BMC
Pediatr 2018;18:44. Crossref
18. Sümpelmann R, Becke K, Crean P, et al. European consensus statement for intraoperative fluid therapy in
children. Eur J Anaesthesiol 2011;28:637-90. Crossref
19. Sümpelmann R, Becke K, Zander R, Witt L. Perioperative
fluid management in children: can we sum it all up now?
Curr Opin Anesthesiol 2019;32:384-91. Crossref
20. Mahajan V, Sajan SS, Sharma A, Kaur J. Ringer’s lactate vs
normal saline for children with acute diarrhea and severe
dehydration—a double blind randomized controlled trial.
Indian Pediatr 2012;49:963-8. Crossref
21. Kartha GB, Rameshkumar R, Mahadevan S. Randomized
double-blind trial of ringer lactate versus normal saline
in pediatric acute severe diarrheal dehydration. J Pediatr
Gastroenterol Nutr 2017;65:621-6. Crossref
22. Fuhrman DY, Kellum JA. Hyperchloremic IV solutions:
have we seen enough?…or “still good medicine?” Pediatr
Crit Care Med 2018;19:171-2. Crossref
23. Kannan L, Lodha R, Vivekanandhan S, Bagga A, Kabra SK,
Kabra M. Intravenous fluid regimen and hyponatraemia
among children: a randomized controlled trial. Pediatr
Nephrol 2010;25:2303-9. Crossref
24. Friedman JN, Beck CE, DeGroot J, et al. Comparison of
isotonic and hypotonic intravenous maintenance fluids: a
randomized clinical trial. JAMA Pediatr 2015;169:445-51. Crossref
25. McNab S, Duke T, South M, et al. 140 mmol/L of sodium
versus 77 mmol/L of sodium in maintenance intravenous
fluid therapy for children in hospital (PIMS): a randomised
controlled double-blind trial. Lancet 2015;385:1190-7. Crossref
26. Pemde HK, Dutta AK, Sodani R, Mishra K. Isotonic
intravenous maintenance fluid reduces hospital acquired
hyponatremia in young children with central nervous
system infections. Indian J Pediatr 2015;82:13-8. Crossref
27. Shamim A, Afzal K, Ali SM. Safety and efficacy of isotonic
(0.9%) vs. hypotonic (0.18%) saline as maintenance
intravenous fluids in children: a randomized controlled
trial. Indian Pediatr 2014;51:969-74. Crossref
28. Golshekan K, Badeli H, Miri M, et al. Suitable intravenous
fluid for preventing dysnatremia in children with
gastroenteritis: a randomized clinical trial. J Renal Inj Prev
2016;5:69-73. Crossref
29. Flores Robles CM, Cuello Garcia CA. A prospective trial
comparing isotonic with hypotonic maintenance fluids for
prevention of hospital-acquired hyponatraemia. Paediatr
Int Child Health 2016;36:168-74. Crossref
30. Torres SF, Iolster T, Schnitzler EJ, et al. Hypotonic and isotonic intravenous maintenance fluids in hospitalised
paediatric patients: a randomised controlled trial. BMJ
Paediatr Open 2019;3:e000385. Crossref
31. Bagri NK, Saurabh VK, Basu S, Kumar A. Isotonic versus
hypotonic intravenous maintenance fluids in children: a
randomized controlled trial. Indian J Pediatr 2019;86:1011-6. Crossref
32. Kumar M, Mitra K, Jain R. Isotonic versus hypotonic
saline as maintenance intravenous fluid therapy in children
under 5 years of age admitted to general paediatric wards:
a randomised controlled trial. Paediatr Int Child Health
2020;40:44-9. Crossref
33. Children’s Hospital Colorado. Clinical pathway:
intravenous fluid therapy. Available from: https://
www.childrenscolorado.org/globalassets/healthcare-professionals/
clinical-pathways/intravenous-fluid-therapy.pdf. Accessed 25 May 2020.
34. Weinberg L, Collins N, Van Mourik K, Tan C, Bellomo R. Plasma-Lyte 148: a clinical review. World J Crit Care Med
2016;5:235-50. Crossref
35. McNab S, Ware RS, Neville KA, et al. Isotonic versus
hypotonic solutions for maintenance intravenous fluid
administration in children. Cochrane Database Syst Rev
2014;(12):CD009457. Crossref
36. Yung M, Keeley S. Randomised controlled trial of intravenous maintenance fluids. J Paediatr Child Health
2009;45:9-14. Crossref
37. Neville KA, Sandeman DJ, Rubinstein A, Henry GM,
McGlynn M, Walker JL. Prevention of hyponatremia
during maintenance intravenous fluid administration: a
prospective randomized study of fluid type versus fluid
rate. J Pediatr 2010;156:313-9.e1-2. Crossref
38. Engorn B, Flerlage J, editors. The Harriet Lane Handbook:
A Manual for Pediatric House Officers 20th ed. Philadelphia
(PA): Elsevier; 2015: 248.
39. Kaptein EM, Sreeramoju D, Kaptein JS, Kaptein MJ.
A systematic literature search and review of sodium
concentrations of body fluids. Clin Nephrol 2016;86:203-28. Crossref
40. Children’s Hospital of Philadelphia. Inpatient clinical
pathway for children who require continuous
administration of IV fluids. Available from: https://www.chop.edu/clinical-pathway/fluid-administration-continuous-iv-clinical-pathway. Accessed 25 May 2020.