© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE CME
Utility of cardiac magnetic resonance imaging in
troponin-positive chest pain with non-obstructive coronary arteries: literature review
Jonan CY Lee, FRCR, FHKAM (Radiology)1; Jeanie B Chiang, FRCR, FHKAM (Radiology)1; PP Ng, MB, ChB1; Boris CK Chow, MB, BS, FRCR1; YW Cheng, MRCP (UK), FHKAM (Medicine)2; CY Wong, MRCP (UK), FHKAM (Medicine)2
1 Department of Radiology and Imaging, Queen Elizabeth Hospital, Hong Kong
2 Department of Medicine, Queen Elizabeth Hospital, Hong Kong
Corresponding author: Dr Jonan CY Lee (jonanleecy@yahoo.com)
Abstract
There is no general consensus on the investigation
and subsequent management of patients presenting
with acute chest pain and elevated cardiac troponin
levels, but with non-obstructive coronary arteries
on angiography. Recent technological advances in
cardiac magnetic resonance imaging have aided in the
understanding of the underlying pathophysiology,
allowing accurate diagnosis, prognostic information,
and guidance for management in these patients.
This article reviews the evidence supporting the
usefulness of cardiac magnetic resonance imaging in
patients with acute chest pain and elevated cardiac
troponin levels, but with non-obstructive coronary
arteries, and offers insights into the role and future development of this imaging modality in this disease.
Introduction
Patients presenting with acute coronary syndrome
require immediate management with coronary
angiography to identify the culprit coronary stenosis.1 2
A small subset of patients with suspected acute
coronary syndrome may have angiographically non-obstructive
coronary arteries, termed myocardial
infarction with non-obstructive coronary arteries
(MINOCA).3 Myocardial infarction with non-obstructive
coronary arteries is indistinguishable in
its clinical presentation from myocardial infarction
with coronary artery disease. The normal coronary
angiography results pose a dilemma to the managing
physician because the underlying aetiology is not
immediately apparent. Arriving at a diagnosis is
challenging, with significant implications regarding
patients’ prognosis, management, and subsequent
follow-up.
Myocardial infarction with non-obstructive
coronary arteries is a distinct clinical entity with
a prevalence of 6% (95% confidence interval
[CI], 5%-7%)3 that deserves further meticulous
investigation. Despite having non-obstructive
coronary arteries, patients with MINOCA have an
increased risk of experiencing major cardiovascular
events (MACE) including death. Pasupathy et al3
reported 4.7% annual mortality in this group
of patients, which is lower than for myocardial
infarction with coronary artery disease (6.7%) but
much higher than in patients with stable chest pain
(0.2% annual mortality).
Causes of MINOCA include acute myocardial
infarction (AMI) with spontaneous recanalisation,
coronary vasospasm, acute myocarditis, takotsubo
cardiomyopathy, and other cardiomyopathies.4
Distinguishing between ischaemic and non-ischaemic
aetiologies is crucial in patients presenting
with MINOCA, in order to tailor treatments
accordingly, such as dual antiplatelet therapy
and other secondary preventive medications for
myocardial infarction,4 or heart failure medications
for myocarditis or cardiomyopathies.
Cardiac magnetic resonance (CMR) imaging
has been increasingly recognised as a first-line
imaging modality in the management of patient
presenting with MINOCA, to detect the aetiology in
a timely manner. High-resolution cardiac images are
acquired with tissue characterisation using different
MR sequences.
The referral rate of CMR imaging for MINOCA
has been low, with only 3% of all eligible patients
undergoing further testing by CMR imaging in a
retrospective study between 2000 and 2016.5 This is
expected to change with the widespread availability
and improved image quality of CMR imaging.
This review aims to summarise the current
evidence regarding the use of CMR imaging in
patients presenting with MINOCA, to demonstrate
its use in various clinical scenarios, and to identify
areas for future research. In particular, we review the
optimal timing of CMR imaging. We also examine
how CMR imaging may change or confirm the aetiology, offers prognostic information, and change management strategy.
We reviewed the medical literature in the
PubMed database and Google Scholar, using the
key terms ‘MINOCA’, ‘myocardial infarction with
non-obstructive coronary arteries’, ‘troponin-positive
acute chest pain’, ‘non-obstructive coronary
arteries’, ‘cardiac magnetic resonance’, ‘myocarditis’,
‘acute myocardial infarction’ and ‘takotsubo
cardiomyopathy’, for studies published up to April
2020. There was no language restriction. Abstracts
were reviewed to determine their relevance to the
aim of our review. Case reports and papers with
unclear or inappropriate statistical methods were
excluded. The discussion is based on, but not limited
to, the search terms.
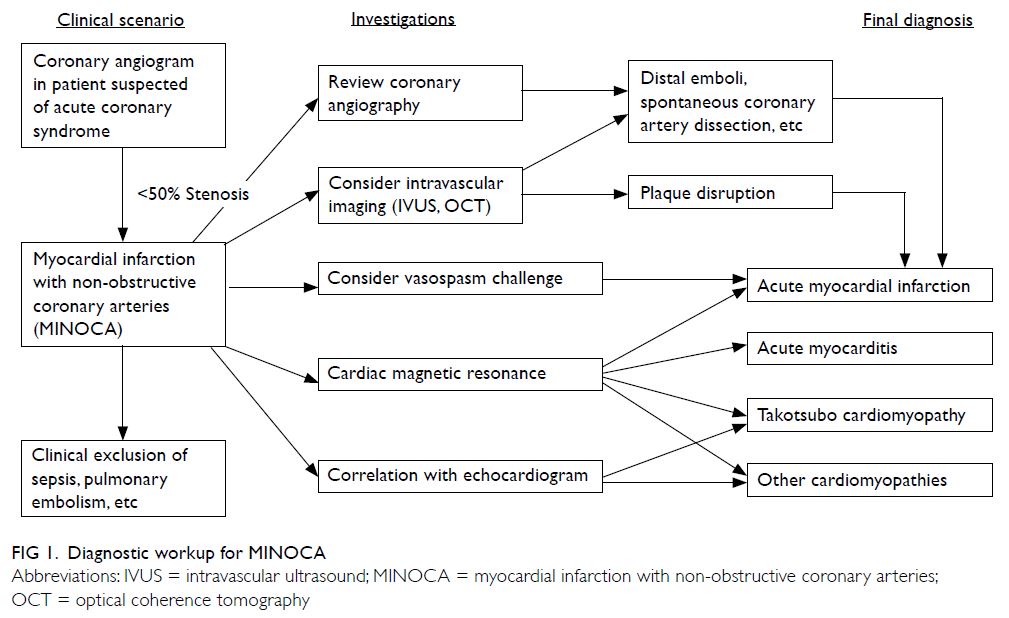
Definition
According to the European Society of Cardiology
working group positional paper6 and the scientific
statement from American Heart Association,7
MINOCA is a distinct clinical syndrome characterised
by evidence of AMI,8 but with no evidence of
obstructive coronary artery disease on angiography
(stenosis <50% diameter in a major epicardial
vessel). The term MINOCA refers to ischaemic-related
coronary disorders, namely plaque rupture,
coronary vasospasm, microvascular dysfunction,
distal embolisation, and coronary artery dissection.
The American Heart Association states that it is
imperative to exclude (a) clinically overt causes for
elevated troponin (eg, sepsis, pulmonary embolism),
(b) clinically over-looked obstructive disease, and (c)
non-ischaemic disease that can mimic myocardial
infarction (eg, myocarditis). In clinical practice,
however, exclusion of non-ischaemic mechanism is
often not straightforward. Elevated cardiac troponin
levels signify myocardial injury, but the marker is
non-specific for the underlying pathophysiological
mechanism. For instance, acute myocarditis and
takotsubo cardiomyopathy may present as MINOCA
and may sometimes be even more frequent than
ischaemic causes.9 Other non-cardiac causes such
as pulmonary embolism or tumour infiltration may
also present as MINOCA.6
Recently, the term troponin-positive chest pain
with non-obstructive coronary arteries (TpNOCA)
has been proposed to encompass all patients
with ischaemic causes as well as non-ischaemic
myocardial disorders and non-cardiac diseases.10 The
Dutch ACS working group suggested that the term
MINOCA can be understood as either myocardial
infarction or myocardial injury with non-obstructive
coronary arteries.11 Given the numerous underlying
possibilities, a detailed diagnostic workup is required
for patient presenting with a working diagnosis of
MINOCA (Fig 1).
Cardiac magnetic resonance imaging protocol
A targeted CMR imaging protocol tailored to the investigation of MINOCA should require no more
than 30 to 40 minutes to perform and is feasible
in most patients except the most critically ill. The
goal of CMR imaging is to assess cardiac motion
and characterise myocardial tissue with full left
ventricular coverage, to detect myocardial oedema
and necrosis for the diagnoses of various disorders,
in particular myocarditis and myocardial infarction.
Commonly performed CMR imaging sequences are
detailed in the online supplementary Appendix.
Myocardial perfusion assessment with
pharmacological stress (eg, adenosine) to evaluate
reversible perfusion defects is seldom required
in patients with MINOCA except for specific
indications such as evaluation of ischaemic extent,
and may be contra-indicated in patients with AMI.12
Therefore, this evaluation is not recommended as
part of the routine assessment.
Timing of cardiac magnetic resonance
imaging
Ideally, CMR imaging should be performed as soon
as possible to identify oedema and acute wall motion
abnormalities. Although CMR imaging is typically
performed after 1 to 4 weeks, the diagnostic value for
patients with MINOCA improves significantly when
performed within 2 weeks of acute presentation.
Studies with longer times to CMR imaging generally
show lower sensitivity for demonstrating pathology.
One study showed that performing CMR imaging
within 2 weeks allowed an underlying cause to
be identified in a higher percentage of the study
population than if CMR imaging was performed
after 2 weeks (82% vs 54%, respectively).13 While
the Dutch ACS working group recommends CMR
imaging within 4 weeks of presentation,11 a stricter
timeframe of performing CMR imaging within
1 week has been suggested by Ferreira et al.4 The local practice may depend on availability of imaging resources.
Differential diagnoses shown in cardiac
magnetic resonance in patients presenting
with myocardial infarction with non-obstructive
coronary arteries
In a recent study by Dastidar et al,9 the predominant
underlying causes of TpNOCA on CMR imaging
in 388 consecutive patients were AMI (25%),
myocarditis (25%), and cardiomyopathy (25%),
although the median time from clinical presentation
to CMR imaging was 37 days. In a recent study by
Bhatia et al5 involving 215 patients, myocarditis
(32%) was the most common cause, followed by
AMI (22%), cardiomyopathy (20%) and takotsubo
cardiomyopathy (9%). The strength of the study was
the short time interval from clinical presentation
to CMR imaging (median: 3.6 days), which could
explain the higher proportion of CMR imaging
studies resulting in positive diagnosis and the
higher incidence of acute myocarditis and takotsubo
cardiomyopathy, in which CMR imaging findings
may be transient.14 Overall, CMR imaging can
provide a diagnosis in 30% to 90% of patients, as
shown in several studies.5 9 15 16 17 18 19 20 21 22 The main reasons for
the wide range in diagnostic rates among different
studies are likely related to the timing of the CMR
imaging, heterogeneity of the patient population,
different referral patterns and imaging sequences and non-standardised diagnostic criteria. Studies
have also shown that CMR imaging results in a
change in clinical diagnosis in more than half of
patients,20 23 and a change in management in 32% to
42%23 24 of patients.
Takotsubo cardiomyopathy
Takotsubo cardiomyopathy (Fig 2), also known as
stress-induced cardiomyopathy or apical ballooning
syndrome, is a reversible cardiomyopathy induced
by extreme physical or emotional stress.25 26 27
However, in some patients, no stressful trigger is
identified. The exact pathophysiology of takotsubo
cardiomyopathy is unclear; however, some postulate
that the underlying pathophysiology is related
to microvascular vasoreactivity25 or hormonal
disturbances.26 Previously considered a benign
condition, an arrhythmogenic risk and increased
cardiac mortality are increasingly recognised in
patients with takotsubo cardiomyopathy.27 In one
study, the prevalence of takotsubo cardiomyopathy
in patients undergoing CMR imaging was as high
as 27%.20 Takotsubo cardiomyopathy is diagnosed
according to the proposed Mayo Clinic criteria.28
Because these criteria do not focus on the role of
CMR imaging, an update in 2016 by the Heart Failure
Association of the European Society of Cardiology
endorsed the use of CMR imaging for its excellent
depiction of right and left ventricular regional wall
motion abnormalities and myocardial oedema.29
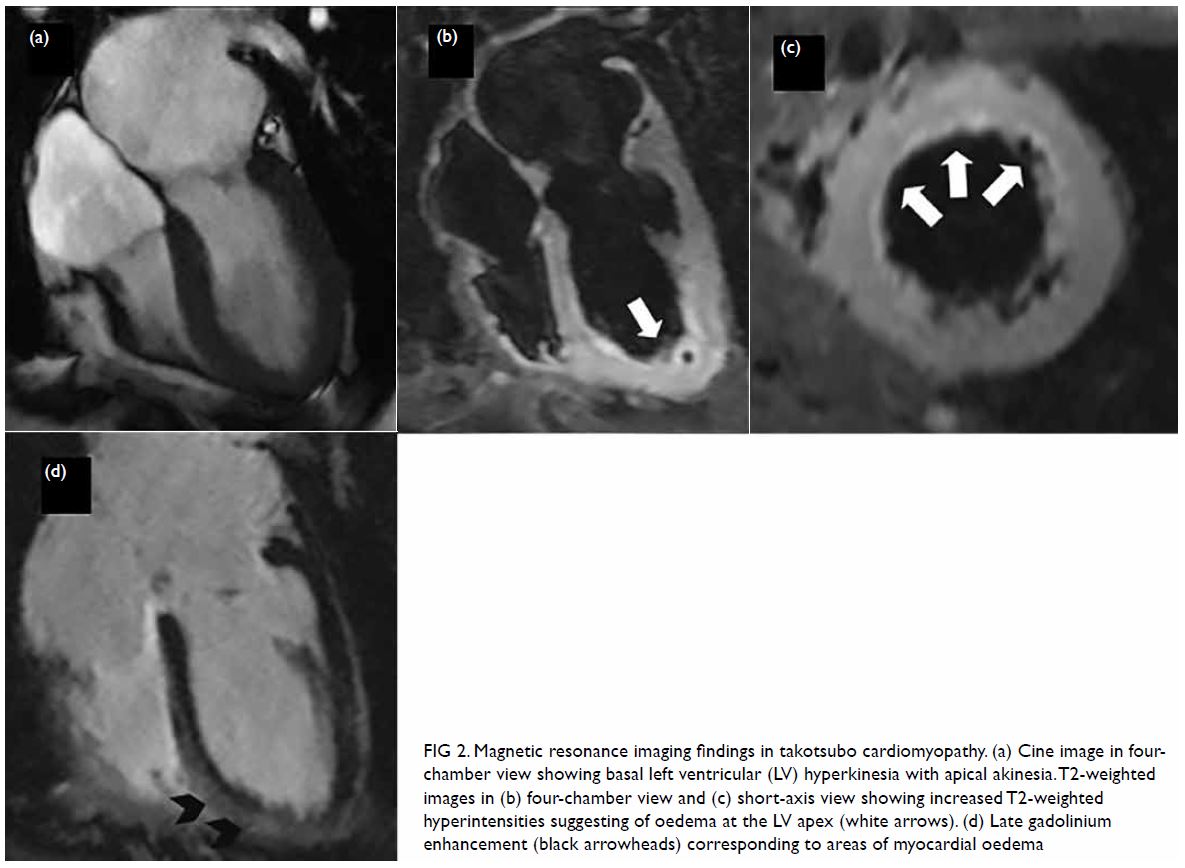
Figure 2. Magnetic resonance imaging findings in takotsubo cardiomyopathy. (a) Cine image in fourchamber view showing basal left ventricular (LV) hyperkinesia with apical akinesia. T2-weighted images in (b) four-chamber view and (c) short-axis view showing increased T2-weighted hyperintensities suggesting of oedema at the LV apex (white arrows). (d) Late gadolinium enhancement (black arrowheads) corresponding to areas of myocardial oedema
On CMR cine imaging, takotsubo
cardiomyopathy has a typical appearance of mid-cavity
to apical akinesia with sparing of basal
segments. Although these findings can also be
seen in echocardiography and left ventricular
angiography, the ability of CMR imaging to assess
areas of myocardial oedema and late gadolinium
enhancement (LGE), as well as to exclude alternative
diagnoses (eg, AMI), makes this an important
modality when assessing takotsubo cardiomyopathy.
Myocardial oedema (as evidenced using short tau
inversion recovery or T2 mapping techniques)
on CMR images correlates with acute myocardial
inflammation30 and electrographic pattern/repolarisation indices31 in takotsubo cardiomyopathy.
The presence of LGE is believed to be transient
rather than irreversible.27 32 33 Another study showed
that LGE in the acute phase was associated with
acute cardiogenic shock, higher peak creatine kinase
levels, and delayed recovery.34 Neil et al35 found that
the extent of the increase in T2-weighted signal
intensity correlated with myocardial strain and the release of both catecholamines and N-terminal pro-B-type natriuretic peptide.
Although no specific treatment is currently available, and spontaneous and complete recovery is
often expected, Dastidar et al9 showed that mortality
in patients with takotsubo cardiomyopathy can be
as high as 15% over 3 years, rejecting the notion
that this is an entirely benign condition. More
studies exploring the underlying mechanism and
management strategy for takotsubo cardiomyopathy
are required.
Acute myocarditis
Acute myocarditis (Fig 3) accounts for 15% to 81%
of CMR imaging diagnoses in multiple studies.
There are myriad causes of acute myocarditis,
including viral infections, autoimmune disease,
and toxins.36 Patients’ clinical courses vary and
range from complete recovery to progression to
chronic myocarditis and dilated cardiomyopathy.
Endomyocardial biopsy remains the gold standard
for diagnosing acute myocarditis, although its use is declining because of its invasiveness and the
possibility of sampling error.37 A previous study
has validated CMR imaging results compared
with endomyocardial biopsy38; CMR-guided
endomyocardial biopsy can improve the diagnostic
rate.39 40
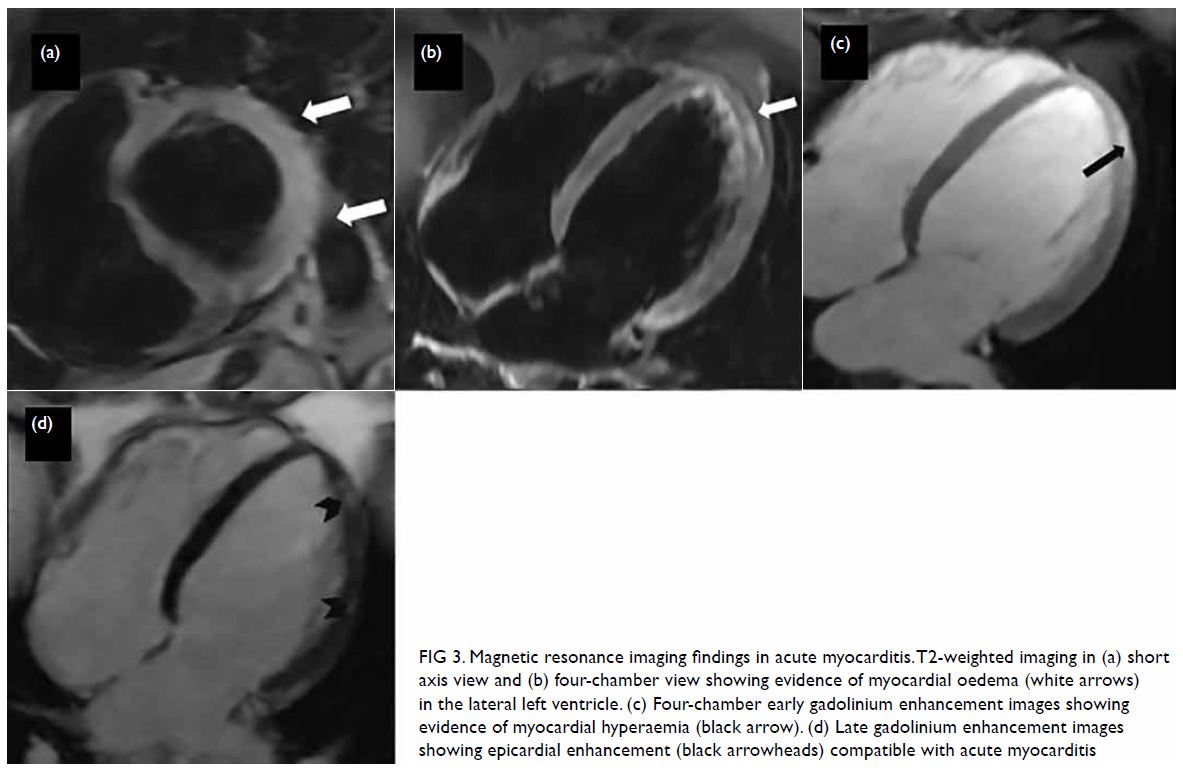
Figure 3. Magnetic resonance imaging findings in acute myocarditis. T2-weighted imaging in (a) short axis view and (b) four-chamber view showing evidence of myocardial oedema (white arrows) in the lateral left ventricle. (c) Four-chamber early gadolinium enhancement images showing evidence of myocardial hyperaemia (black arrow). (d) Late gadolinium enhancement images showing epicardial enhancement (black arrowheads) compatible with acute myocarditis
The CMR diagnosis of acute myocarditis has
been made according to the original Lake Louise
criteria, which were established in 2009.41 These
criteria are based on the presence of at least two of
three CMR imaging findings: myocardial oedema
on T2-weighted images, hyperaemia and capillary
leak on EGE, and fibrosis/necrosis on LGE. These
criteria have a diagnostic accuracy of 78% for
acute myocarditis. However, co-existing skeletal
inflammation may lead to false-negative results in
T2 short tau inversion recovery/early gadolinium
enhancement images.42 With the development of
parametric mapping, T1 mapping can establish the
diagnosis of myocarditis, even without contrast
injection for LGE.43 T1 mapping as an individual
parameter was found to have superior diagnostic
performance for detecting myocarditis compared
with T2-weighted oedema imaging.42 More recently,
a Journal of the American College of Cardiology
scientific expert panel updated the use of CMR imaging in myocarditis to include parametric
mapping based on at least one T2-based criterion
(global or regional increase in myocardial T2
relaxation time or an increased signal intensity in T2-weighted CMR images), with at least one T1-based
criterion (increased myocardial T1, extracellular
volume or LGE).44 The inclusion of global or
regional T1 or T2 myocardial values is expected to
improve the diagnostic accuracy of CMR imaging
compared with the original Lake Louise criteria.
Extracellular volume measurements can also be
obtained after contrast administration, adjusting for
individual variation in the haematocrit value that
may affect the result. The presence of both T2- and
T1-based criteria is diagnostic of acute myocardial
inflammation, while having only one criterion
may still support the diagnosis in an appropriate
clinical scenario, albeit with less specificity. The
updated Lake Louise criteria have been validated by
Luetkens et al45 to have better sensitivity than the
original Lake Louise criteria (88% vs 73%, P=0.031),
with a similar high specificity of 96%.
In addition to diagnosing acute myocarditis, CMR imaging findings have prognostic implications
and can help guide patient management. Grun et al46
indicated that LGE was the best independent predictor of all-cause mortality and of cardiac
mortality in 222 consecutive patients with biopsy-proven
viral myocarditis. A recent systematic
review and meta-analysis by Yang et al47 showed
that LGE in patients with myocarditis or suspected
myocarditis was significantly associated with MACE
(pooled odds ratio=4.57, 95% confidence interval
[CI]=2.18-9.59; P<0.001), regardless of the left
ventricular ejection fraction. A study by Grani et al48
showed that both the pattern and extent of LGE were
significantly associated with MACE. Aquaro et al49
showed that the prognostic value of CMR imaging
extends beyond the acute phase, with the presence of
LGE with oedema at 6 months being an independent
predictor of adverse cardiac events and associated
with worse prognosis, especially mid-wall septal
patterns in LGE.
More studies are required to determine whether
CMR imaging can help differentiate the subtypes of
myocarditis (viral, eosinophilic, autoimmune and
giant cell myocarditis).44
Acute myocardial infarction
Acute myocardial infarction (Fig 4) was either the most common or second most common aetiology
detected by CMR imaging in previous studies,
ranging from 11% to 26%.5 9 The underlying
pathophysiological mechanisms included plaque
disruption with spontaneous recanalisation, distal
embolisation, coronary vasospasm, dissection, or
distal small branch disease. On CMR imaging, a
classic subendocardial or transmural LGE pattern
corresponding to the coronary artery territory, with
or without microvascular obstruction, is diagnostic
of myocardial infarction. If an infarct is seen, it is
essential to review the coronary angiographic images
for subtle missed obstructive lesions or coronary
artery dissection, and to rule out vasospasm or distal
embolisation. Further investigations may depend on
clinical suspicion and local practice, and may include
intravascular imaging such as optical coherence
tomography and intravascular ultrasonography for
plaque assessment, provocative tests for coronary
vasospasm, echocardiography to identify an embolic
source (eg, patent foramen ovale) and thrombophilia
screening for hypercoagulable disorders. In Asian
populations, vasospastic angina is particularly
common and should be carefully managed.50 Drugs
such as cocaine are well-documented causes of
coronary vasospasm and careful elucidation of
history is required.
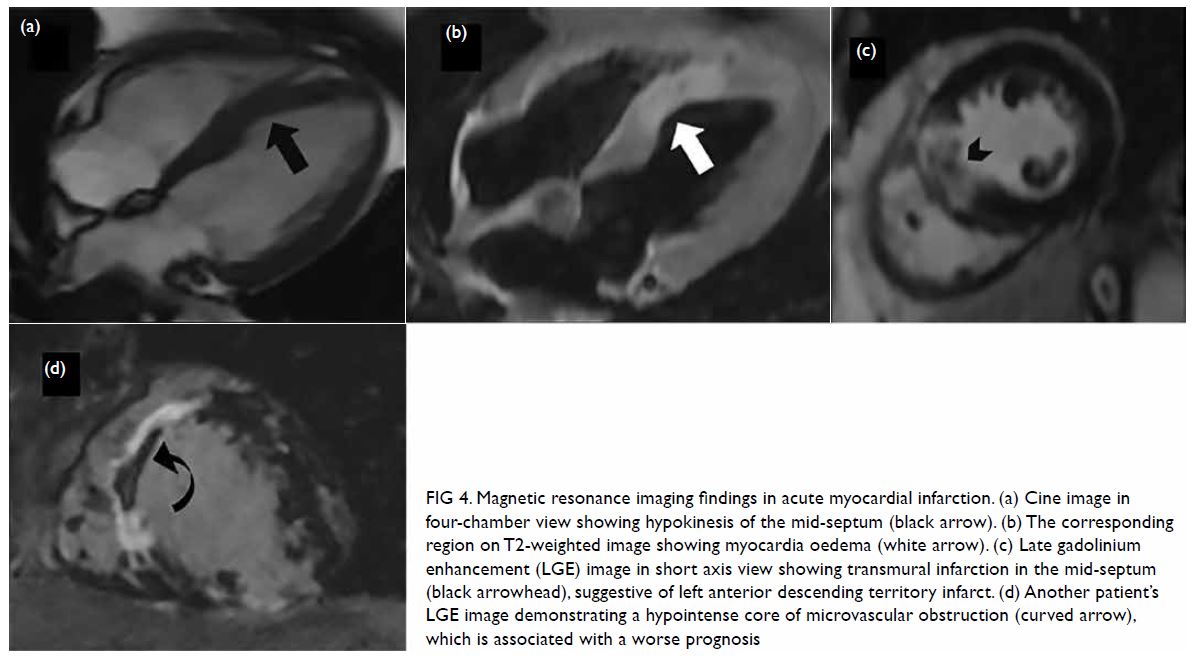
Figure 4. Magnetic resonance imaging findings in acute myocardial infarction. (a) Cine image in four-chamber view showing hypokinesis of the mid-septum (black arrow). (b) The corresponding region on T2-weighted image showing myocardia oedema (white arrow). (c) Late gadolinium enhancement (LGE) image in short axis view showing transmural infarction in the mid-septum (black arrowhead), suggestive of left anterior descending territory infarct. (d) Another patient’s LGE image demonstrating a hypointense core of microvascular obstruction (curved arrow), which is associated with a worse prognosis
In addition to providing a diagnosis, CMR
imaging can also assess myocardial oedema and
myocardium at risk in the acute phase to calculate the
salvageable area, as well as to assess complications
of AMI, such as pseudoaneurysms or intra-cardiac
thrombus. Both the presence of a scar and the
quantifiable extent of the infarct on LGE have been shown to carry prognostic significance in AMI for
predicting morbidity and mortality.51 52 The presence
of microvascular obstruction is also associated with
a worse prognosis.53 T1 mapping and extracellular
volume measurement may be able to differentiate
between acute and chronic myocardial infarction.54
Non-ischaemic cardiomyopathies
Hypertrophic cardiomyopathy and dilated
cardiomyopathy are the most common forms of
non-ischaemic cardiomyopathy presenting with
MINOCA (Fig 5). These cardiomyopathies can be
diagnosed using CMR imaging according to their
morphology and LGE patterns.55 The prevalence
of non-ischaemic cardiomyopathies in MINOCA
varies widely in the literature, and it is unclear
whether affected patients were excluded in some
studies. Bhatia et al5 showed the highest prevalence
of cardiomyopathy among studies that included
affected patients, with a prevalence of 20%, making
cardiomyopathy the third most common aetiology in
MINOCA. A recent study by Dastidar et al9 showed
that cardiomyopathy had the worst prognosis among
all diagnoses.
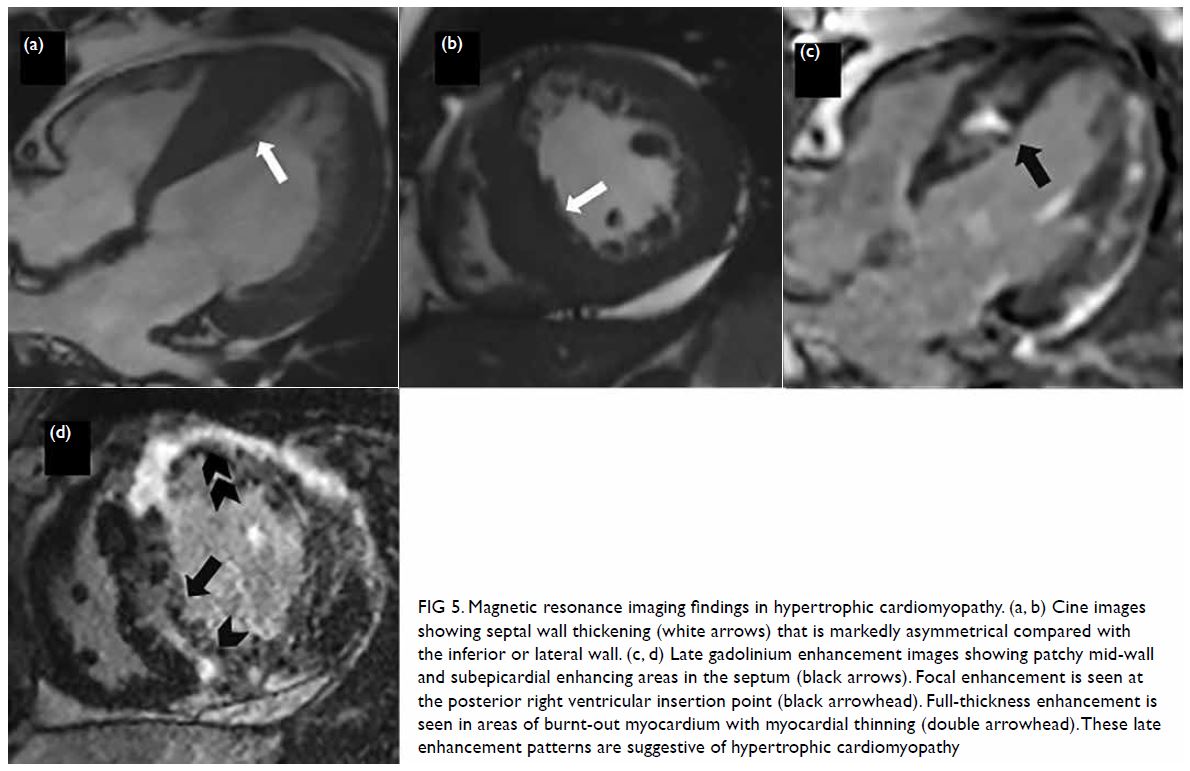
Figure 5. Magnetic resonance imaging findings in hypertrophic cardiomyopathy. (a, b) Cine images showing septal wall thickening (white arrows) that is markedly asymmetrical compared with the inferior or lateral wall. (c, d) Late gadolinium enhancement images showing patchy mid-wall and subepicardial enhancing areas in the septum (black arrows). Focal enhancement is seen at the posterior right ventricular insertion point (black arrowhead). Full-thickness enhancement is seen in areas of burnt-out myocardium with myocardial thinning (double arrowhead). These late enhancement patterns are suggestive of hypertrophic cardiomyopathy
A systematic review by Kuruvilla et al56 showed
that patients with non-ischaemic cardiomyopathy
with LGE had greater all-cause mortality compared
with patients without LGE (odds ratio=3.27;
95% CI=1.94-5.51; P<0.00001).56 In hypertrophic
cardiomyopathy, a meta-analysis57 showed that the
presence of LGE was associated with an increased
risk of sudden cardiac death, heart failure, and
cardiovascular mortality and that the extent of LGE
was also strongly associated with the risk of sudden
cardiac death, suggesting that quantifying LGE is an
important tool for risk stratification.
The growing use of parametric mapping will no doubt further enhance the diagnostic capability of
CMR imaging in non-ischaemic cardiomyopathies.54
Normal/inconclusive cardiac magnetic resonance
Cardiac magnetic resonance may sometimes not
reveal a specific diagnosis, the proportion of which
depends on the timing of CMR imaging as well as
patients’ demographics. Patients with negative CMR
imaging findings typically have a lower troponin
level.20 Occasionally, an infarct may be too small to be
visualised by conventional LGE sequences.58 Negative
CMR imaging findings do not exclude MINOCA.
Regardless of whether the underlying cause is
identified, the absence of positive CMR imaging
findings is associated with a better prognosis.9
Managing patients with myocardial
infarction with non-obstructive coronary
arteries
Limited guidelines exist regarding the current
recommended management of patients with MINOCA, and the management algorithm differs
in different centres. Treatment obviously depends
on the underlying diagnosis, if identified. In patients
without an apparent cause, even by CMR imaging,
evidence-based therapies are lacking. Recently,
aspirin, statins and calcium channel blockers have
been proposed as routine medical treatment in
patients with no clear aetiology for elevated troponin
on CMR images, to potentially treat underlying
thromboembolism, coronary plaque disruption and
coronary artery vasospasm.6 The evidence for the use
of beta-blocker is conflicting.59 60 The confirmation
of the benefits of these therapies would require a
multicentre randomised controlled trial.
Questions to be addressed
There is a distinct lack of published studies evaluating
patients of Asian descent with MINOCA, for whom
the local disease spectrum with CMR imaging and
the prognostic significance may differ from studies
evaluating patients from Western countries, because
of differences in the underlying risk factors. It is
still unclear in current studies whether performing
CMR imaging improves patient outcomes regarding
shortening hospital stay, preventing re-admission
and lowering MACE and mortality rates. This
hypothesis requires validation in further studies
in a large patient cohort, with longer follow-up of
clinical outcomes. Further studies are also needed
to evaluate the relationship between troponin and the extent of LGE, the optimal management pathway
and secondary prevention, as well as the role of long-term
imaging surveillance to guide management in
patients with MINOCA.
Future directions
With the emergence of novel parametric mapping
techniques, namely T1/T2 mapping and extracellular
volume measurement, the sensitivity of CMR
imaging is expected to improve, as most previous
studies did not use T1 and T2 mapping. The optimal
mapping techniques and post-processing methods
are still being determined,61 after which the capability
of CMR imaging for diagnosis and prognostication
can be further enhanced, providing a better
understanding of the underlying pathophysiology
in MINOCA. A gadolinium-free or LGE-free
protocol combining T2-based CMR imaging with
T1 mapping holds significant promise, especially
for patients contra-indicated for gadolinium, but
further studies are required before this approach can
be routinely implemented. Further developments in
CMR imaging techniques, such as three-dimensional
free-breathing high-resolution LGE,58 can lead to a
higher rate of definitive myocardial LGE evaluation,
thereby reducing the false-negative rate in MINOCA
diagnosis. Dedicated rapid CMR imaging protocols
or compressed sensing cine can shorten scanning
times and permit acquiring diagnostic CMR imaging
information even in critically ill patients.
In conclusion, troponin-positive chest pain
with non-obstructive coronary arteries should be
recognised as a distinct clinical entity that deserves an
active search for the underlying cause and a detailed
management plan. The absence of obstructive
disease on angiography does not necessarily
exclude AMI. When performed early in the disease
course, CMR imaging is the ideal non-invasive
adjunct to conventional cardiac investigations in
patients presenting as MINOCA. Cardiac magnetic
resonance should be routinely used in these patients
for diagnosis and risk stratification to guide further
therapy.
Author contributions
Concept or design: JCY Lee.
Acquisition of data: All authors.
Analysis or interpretation of data: JCY Lee, JB Chiang, PP Ng, BCK Chow.
Drafting of the manuscript: JCY Lee, JB Chiang, YW Cheng, CY Wong.
Critical revision of the manuscript for important intellectual content: JCY Lee, YW Cheng, CY Wong.
Acquisition of data: All authors.
Analysis or interpretation of data: JCY Lee, JB Chiang, PP Ng, BCK Chow.
Drafting of the manuscript: JCY Lee, JB Chiang, YW Cheng, CY Wong.
Critical revision of the manuscript for important intellectual content: JCY Lee, YW Cheng, CY Wong.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
The authors have disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patients were treated in accordance with the Declaration of Helsinki. The patients provided written informed consent
for all procedures.
References
1. Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for
the management of acute coronary syndromes in patients
presenting without persistent ST-segment elevation: Task
Force for the management of acute coronary syndromes
in patients presenting without persistent ST-segment
elevation of the European Society of Cardiology (ESC). Eur
Heart J 2016;37:267-315. Crossref
2. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for
the management of acute myocardial infarction in patients
presenting with ST-segment elevation: The Task Force for
the management of acute myocardial infarction in patients
presenting with ST-segment elevation of the European
Society of Cardiology (ESC). Eur Heart J 2018;39:119-77. Crossref
3. Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected
myocardial infarction and nonobstructive coronary
arteries. Circulation 2015;131:861-70. Crossref
4. Ferreira VM. CMR should be a mandatory test in the
contemporary evaluation of ‘MINOCA’. JACC Cardiovasc
Imaging 2019;12:1983-6. Crossref
5. Bhatia S, Anstine C, Jaffe AS, et al. Cardiac magnetic
resonance in patients with elevated troponin and normal
coronary angiography. Heart 2019;105:1231-6. Crossref
6. Agewall S, Beltrame JF, Reynolds HR, et al. ESC working
group position paper on myocardial infarction with non-obstructive
coronary arteries. Eur Heart J 2017;38:143-53.
7. Tamis-Holland JE, Jneid H, Reynolds HR, et al.
Contemporary diagnosis and management of patients
with myocardial infarction in the absence of obstructive
coronary artery disease: a scientific statement from the
American Heart Association. Circulation. 2019;139:e891-e908. Crossref
8. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Circulation
2018;138:e618-e651. Crossref
9. Dastidar AG, Baritussio A, De Garate E, et al. Prognostic
role of CMR and conventional risk factors in myocardial
infarction with nonobstructed coronary arteries. JACC
Cardiovasc Imaging 2019;12:1973-82. Crossref
10. Pasupathy S, Tavella R, Beltrame JF. Myocardial infarction
with nonobstructive coronary arteries (MINOCA):
the past, present, and future management. Circulation
2017;135:1490-3. Crossref
11. Pustjens TF, Appelman Y, Damman P, et al. Guidelines
for the management of myocardial infarction/injury with
non-obstructive coronary arteries (MINOCA): a position
paper from the Dutch ACS working group. Neth Heart J
2020;28:116-30. Crossref
12. Jo Y, Kim J, Park CH, et al. Guideline for cardiovascular
magnetic resonance imaging from the Korean Society of
Cardiovascular Imaging—part 1: standardized protocol.
Korean J Radiol 2019;20:1313-33. Crossref
13. Dastidar AG, Singhal P, Rodrigues JC, et al. Improved
diagnostic role of CMR in acute coronary syndromes and
unobstructed coronary arteries: the importance of time-to-CMR. J Cardiovasc Magn Reson 2015;17(Suppl 1):O87. Crossref
14. Sechtem U, Seitz A, Ong P. MINOCA: unravelling the
enigma. Heart 2019;105:1219-20. Crossref
15. Assomull RG, Lyne JC, Keenan N, et al. The role of
cardiovascular magnetic resonance in patients presenting
with chest pain, raised troponin, and unobstructed
coronary arteries. Eur Heart J 2007;28:1242-9. Crossref
16. Monney PA, Sekhri N, Burchell T, et al. Acute myocarditis
presenting as acute coronary syndrome: role of early cardiac
magnetic resonance in its diagnosis. Heart 2011;97:1312-8. Crossref
17. Leurent G, Langella B, Fougerou C, et al. Diagnostic
contributions of cardiac magnetic resonance imaging in
patients presenting with elevated troponin, acute chest
pain syndrome and unobstructed coronary arteries. Arch
Cardiovasc Dis 2011;104:161-70. Crossref
18. Mahmoudi M, Harden S, Abid N, et al. Troponin-positive
chest pain with unobstructed coronary arteries: definitive
differential diagnosis using cardiac MRI. Br J Radiol
2012;85:e461-6. Crossref
19. Collste O, Sörensson P, Frick M, et al. Myocardial
infarction with normal coronary arteries is common
and associated with normal findings on cardiovascular magnetic resonance imaging: results from the Stockholm
Myocardial Infarction with Normal Coronaries study. J
Intern Med 2013;273:189-96. Crossref
20. Pathik B, Raman B, Mohd Amin NH, et al. Troponin-positive
chest pain with unobstructed coronary arteries:
incremental diagnostic value of cardiovascular magnetic
resonance imaging. Eur Heart J Cardiovasc Imaging
2016;17:1146-52. Crossref
21. Chopard R, Jehl J, Dutheil J, et al. Evolution of acute
coronary syndrome with normal coronary arteries and
normal cardiac magnetic resonance imaging. Arch
Cardiovasc Dis 2011;104:509-17. Crossref
22. Laraudogoitia Zaldumbide E, Pérez-David E, Larena JA, et al.
The value of cardiac magnetic resonance in patients with
acute coronary syndrome and normal coronary arteries [in
Spanish]. Rev Esp Cardiol 2009;62:976-83. Crossref
23. Dastidar AG, Rodrigues JC, Johnson TW, et al. Myocardial
infarction with nonobstructed coronary arteries: impact of
CMR early after presentation. JACC Cardiovasc Imaging
2017;10(10 Pt A):1204-6. Crossref
24. Gerbaud E, Harcaut E, Coste P, et al. Cardiac magnetic
resonance imaging for the diagnosis of patients presenting
with chest pain, raised troponin, and unobstructed
coronary arteries. Int J Cardiovasc Imaging 2012;28:783-94. Crossref
25. Galiuto L, De Caterina AR, Porfidia A, et al. Reversible
coronary microvascular dysfunction: a common
pathogenetic mechanism in apical ballooning or Tako-Tsubo syndrome. Eur Heart J 2010;31:1319-27. Crossref
26. Pizzino G, Bitto A, Crea P, et al. Takotsubo syndrome and
estrogen receptor genes: partners in crime? J Cardiovasc
Med (Hagerstown) 2017;18:268-76. Crossref
27. Dastidar AG, Frontera A, Palazzuoli A, Bucciarelli-Ducci C.
Takotsubo cardiomyopathy: unravelling the malignant
consequences of a benign disease with cardiac magnetic
resonance. Heart Fail Rev 2015;20:415-21. Crossref
28. Madhavan M, Prasad A. Proposed Mayo Clinic criteria for
the diagnosis of Tako-Tsubo cardiomyopathy and long-term
prognosis. Herz 2010;35:240-3. Crossref
29. Lyon AR, Bossone E, Schneider B, et al. Current state of
knowledge on takotsubo syndrome: a position statement
from the Taskforce on Takotsubo Syndrome of the Heart
Failure Association of the European Society of Cardiology.
Eur J Heart Fail 2016;18:8-27. Crossref
30. Iacucci I, Carbone I, Cannavale G, et al. Myocardial
oedema as the sole marker of acute injury in takotsubo
cardiomyopathy: a cardiovascular magnetic resonance
(CMR) study. Radiol Med 2013;118:1309-23. Crossref
31. Perazzolo Marra M, Zorzi A, Corbetti F, et al. Apicobasal
gradient of left ventricular myocardial edema underlies
transient T-wave inversion and QT interval prolongation
(Wellens’ ECG pattern) in Tako-Tsubo cardiomyopathy.
Heart Rhythm 2013;10:70-7. Crossref
32. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome
(Tako-Tsubo or stress cardiomyopathy): a mimic of acute
myocardial infarction. Am Heart J 2008;155:408-17. Crossref
33. Rolf A, Nef HM, Möllmann H, et al. Immunohistological
basis of the late gadolinium enhancement phenomenon in
tako-tsubo cardiomyopathy. Eur Heart J 2009;30:1635-42. Crossref
34. Naruse Y, Sato A, Kasahara K, et al. The clinical
impact of late gadolinium enhancement in takotsubo
cardiomyopathy: serial analysis of cardiovascular magnetic
resonance images. J Cardiovasc Magn Reson 2011;13:67. Crossref
35. Neil C, Nguyen TH, Kucia A, et al. Slowly resolving
global myocardial inflammation/oedema in Tako-Tsubo
cardiomyopathy: evidence from T2-weighted cardiac MRI.
Heart 2012;98:1278-84. Crossref
36. Kindermann I, Barth C, Mahfoud F, et al. Update on myocarditis. J Am Coll Cardiol 2012;59:779-92. Crossref
37. Leone O, Veinot JP, Angelini A, et al. 2011 Consensus
statement on endomyocardial biopsy from the Association
for European Cardiovascular Pathology and the Society for
Cardiovascular Pathology. Cardiovasc Pathol 2012;21:245-74. Crossref
38. Lurz P, Eitel I, Adam J, et al. Diagnostic performance of CMR
imaging compared with EMB in patients with suspected
myocarditis. JACC Cardiovasc Imaging 2012;5:513-24. Crossref
39. Mahrholdt H, Goedecke C, Wagner A, et al. Cardiovascular
magnetic resonance assessment of human myocarditis:
a comparison to histology and molecular pathology.
Circulation 2004;109:1250-8. Crossref
40. Baccouche H, Mahrholdt H, Meinhardt G, et al. Diagnostic
synergy of non-invasive cardiovascular magnetic resonance
and invasive endomyocardial biopsy in troponin-positive
patients without coronary artery disease. Eur Heart J
2009;30:2869-79. Crossref
41. Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: A
JACC White Paper. J Am Coll Cardiol 2009;53:1475-87. Crossref
42. Ferreira VM, Piechnik SK, Dall’Armellina E, et al. T1
mapping for the diagnosis of acute myocarditis using CMR:
comparison to T2-weighted and late gadolinium enhanced
imaging. JACC Cardiovasc Imaging 2013;6:1048-58. Crossref
43. Ferreira VM, Piechnik SK, Dall’Armellina E, et al. Native
T1-mapping detects the location, extent and patterns
of acute myocarditis without the need for gadolinium
contrast agents. J Cardiovasc Magn Reson 2014;16:36. Crossref
44. Ferreira VM, Schulz-Menger J, Holmvang G, et al.
Cardiovascular magnetic resonance in nonischemic
myocardial inflammation: expert recommendations. J Am
Coll Cardiol 2018;72:3158-76. Crossref
45. Luetkens JA, Faron A, Isaak A, et al. Comparison of
original and 2018 Lake Louise criteria for diagnosis of
acute myocarditis: results of a validation cohort. Radiol
Cardiothorac Imaging 2019;1:e190010. Crossref
46. Grün S, Schumm J, Greulich S, et al. Long-term follow-up
of biopsy-proven viral myocarditis: predictors of mortality
and incomplete recovery. J Am Coll Cardiol 2012;59:1604-15. Crossref
47. Yang F, Wang J, Li W, et al. The prognostic value of late
gadolinium enhancement in myocarditis and clinically
suspected myocarditis: systematic review and meta-analysis.
Eur Radiol 2020;30:2616-26. Crossref
48. Gräni C, Eichhorn C, Bière L, et al. Prognostic value of
cardiac magnetic resonance tissue characterization in risk
stratifying patients with suspected myocarditis. J Am Coll
Cardiol 2017;70:1964-76. Crossref
49. Aquaro GD, Ghebru Habtemicael Y, Camastra G, et al.
Prognostic value of repeating cardiac magnetic resonance in patients with acute myocarditis. J Am Coll Cardiol
2019;74:2439-48. Crossref
50. Beltrame JF, Crea F, Kaski JC, et al. The who, what, why, when, how and where of vasospastic angina. Circ J
2016;80:289-98. Crossref
51. Larose E, Rodés-Cabau J, Pibarot P, et al. Predicting late
myocardial recovery and outcomes in the early hours of
ST-segment elevation myocardial infarction traditional
measures compared with microvascular obstruction,
salvaged myocardium, and necrosis characteristics by
cardiovascular magnetic resonance. J Am Coll Cardiol
2010;55:2459-69. Crossref
52. Yokota H, Heidary S, Katikireddy CK, et al. Quantitative
characterization of myocardial infarction by cardiovascular
magnetic resonance predicts future cardiovascular events
in patients with ischemic cardiomyopathy. J Cardiovasc
Magn Reson 2008;10:17. Crossref
53. Taylor AJ, Al-Saadi N, Abdel-Aty H, Schulz-Menger J,
Messroghli DR, Friedrich MG. Detection of acutely
impaired microvascular reperfusion after infarct
angioplasty with magnetic resonance imaging. Circulation
2004;109:2080-5. Crossref
54. Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood
JP, Plein S. Cardiac T1 mapping and extracellular volume
(ECV) in clinical practice: a comprehensive review. J
Cardiovasc Magn Reson 2016;18:89. Crossref
55. Karamitsos TD, Francis JM, Myerson S, Selvanayagam JB,
Neubauer S. The role of cardiovascular magnetic resonance
imaging in heart failure. J Am Coll Cardiol 2009;54:1407-24. Crossref
56. Kuruvilla S, Adenaw N, Katwal AB, Lipinski MJ, Kramer CM,
Salerno M. Late gadolinium enhancement on cardiac
magnetic resonance predicts adverse cardiovascular
outcomes in nonischemic cardiomyopathy: a systematic
review and meta-analysis. Circ Cardiovasc Imaging
2014;7:250-8. Crossref
57. Weng Z, Yao J, Chan RH, et al. Prognostic value of
LGE-CMR in HCM: a meta-analysis. JACC Cardiovasc
Imaging 2016;9:1392-402. Crossref
58. Lintingre PF, Nivet H, Clément-Guinaudeau S, et al.
High-resolution late gadolinium enhancement magnetic
resonance for the diagnosis of myocardial infarction
with nonobstructed coronary arteries. JACC Cardiovasc
Imaging 2020;13:1135-48. Crossref
59. Lindahl B, Baron T, Erlinge D, et al. Medical therapy for
secondary prevention and long-term outcome in patients
with myocardial infarction with nonobstructive coronary
artery disease. Circulation 2017;135:1481-9. Crossref
60. Pelliccia F, Pasceri V, Niccoli G, et al. Predictors of mortality
in myocardial infarction and nonobstructed coronary
arteries: a systematic review and meta-regression. Am J
Med 2020;133:73-83.e4. Crossref
61. Peker E, Gülpınar B, Elhan AH, Erden MI. Diagnostic
accuracy of mapping techniques and postprocessing
methods for acute myocarditis. AJR Am J Roentgenol
2020;215:105-15. Crossref