© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Ketamine-associated nephropathy treated with
renal transplantation: a case report
John SL Leung, MB, BS1; Vincent YK Poon, FRCSEd (Urol)1; Thomas YC Lam, FRCSEd (Urol)1; CK Chan, FRCSEd (Urol)1; Y Chiu, FRCSEd (Urol)1; TY Chu, FRCSEd (Urol)1; Samuel KS Fung, FRCP (Edin), FRCP (Irel)2; WK Ma, FRCSEd (Urol)1
1 Department of Surgery, Princess Margaret Hospital, Hong Kong
2 Department of Medicine and Geriatrics, Princess Margaret Hospital, Hong Kong
Corresponding author: Dr WK Ma (drmawk@gmail.com)
Case report
We present a 37-year-old man who had been on
continuous ambulatory peritoneal dialysis for 8 years
owing to ketamine-associated end-stage renal failure.
He received a cadaveric renal graft in December
2019 at Princess Margaret Hospital, Hong Kong.
He first presented with haematuria and dysuria
in 2004. He had been abusing ketamine steadily for 4
years. Mid-stream urine culture and acid-fast bacillus
culture were negative; urine cytology, renal function,
and ultrasonography of the urinary system were
unremarkable. The patient subsequently defaulted
on investigations and follow-up appointments.
The patient returned in 2010 with more
severe symptoms of ketamine cystitis and reflux
nephropathy. At that time, he was taking 0.3 to 0.6 g
of ketamine by nasal inhalation up to 10 times a day.
He had urinary frequency every 10 minutes and
creatinine was 283 μmol/L (estimated glomerular
filtration rate [eGFR] 25 mL/min/1.73 m2). Flexible
cystoscopy showed cystitis changes; a biopsy of the
urothelium yielded neutrophilic exudates mixed with
fibrin, and fibroblastic stromal reaction. Ultrasound
scan revealed bilateral hydronephrosis and a
thickened bladder wall. Non-contrast computed tomography showed thinning of the renal cortex
and bilateral hydronephrosis as well as thickening
of the ureters, all indicative of ureteric inflammation
(Fig 1). Repeat urine cytology, routine culture, and
acid-fast bacillus cultures were all negative.
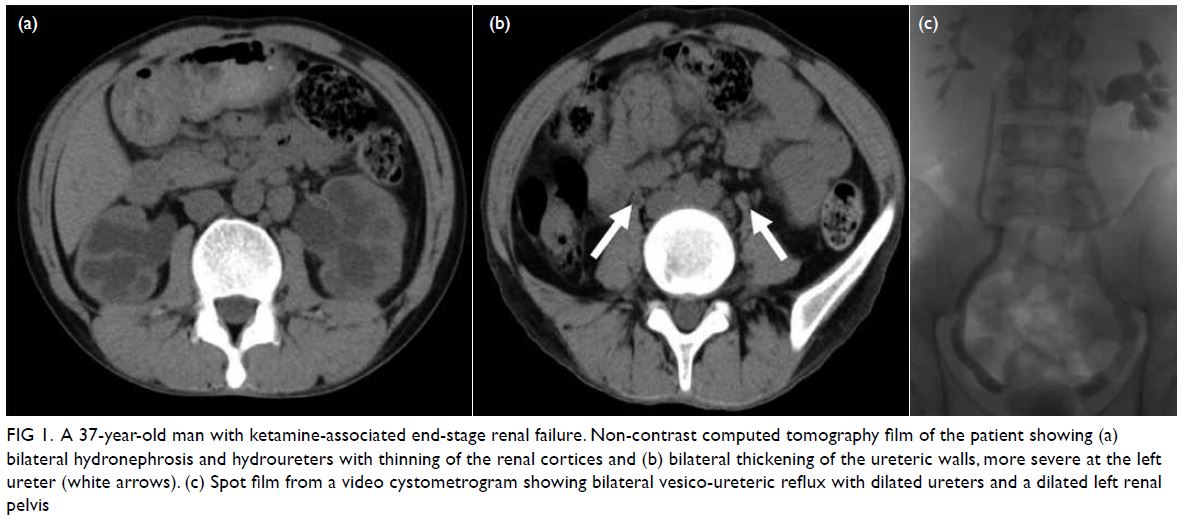
Figure 1. A 37-year-old man with ketamine-associated end-stage renal failure. Non-contrast computed tomography film of the patient showing (a) bilateral hydronephrosis and hydroureters with thinning of the renal cortices and (b) bilateral thickening of the ureteric walls, more severe at the left ureter (white arrows). (c) Spot film from a video cystometrogram showing bilateral vesico-ureteric reflux with dilated ureters and a dilated left renal pelvis
Video cystometrogram demonstrated typical
features of ketamine cystitis, namely that of a small
and contracted bladder: the bladder capacity was
25 mL; first desire to void was at 14 mL; detrusor
instability occurred at a Pdet of 22 cmH2O (Fig 2).
Additionally, bilateral grade III vesico-ureteric reflux
was documented (Fig 1). The patient agreed only to
a urethral catheter and refused upper tract urinary
diversion with percutaneous nephrostomies.
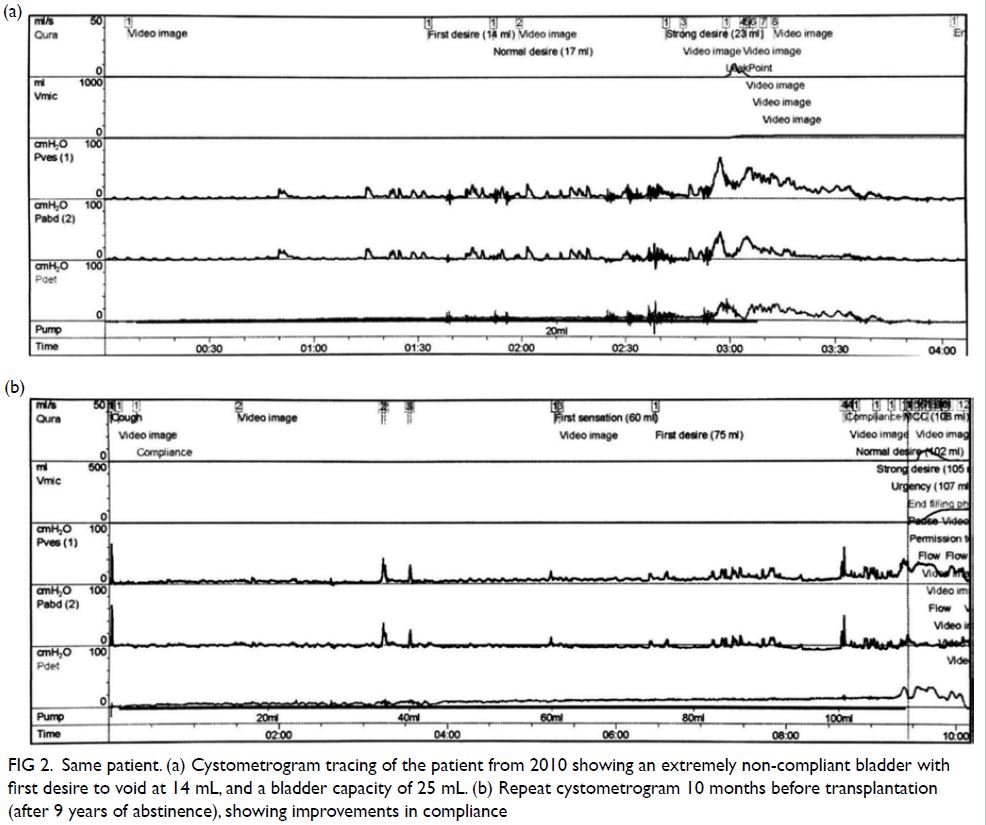
Figure 2. Same patient. (a) Cystometrogram tracing of the patient from 2010 showing an extremely non-compliant bladder with first desire to void at 14 mL, and a bladder capacity of 25 mL. (b) Repeat cystometrogram 10 months before transplantation (after 9 years of abstinence), showing improvements in compliance
He began abstaining from ketamine in 2010
but refused dialysis until 2011 when his creatinine
had reached 1079 μmol/L (eGFR 5 mL/min/1.73 m2).
Annual broad-spectrum drug screening of the
patient’s urine samples was negative for ketamine
and its metabolites since then.
A repeat video cystometrogram in 2013, 3 years after complete abstinence from ketamine, showed
improvements from his first video cystometrogram
in 2010. Bladder capacity had improved to 124 mL;
first desire to void improved to 51 mL. Detrusor overactivity was noted at 120 mL when the Pdet was 30 cmH2O. Only a left grade I vesico-ureteric reflux
was observed.
In 2019, another video cystometrogram
showed improvement in the first desire to void to
75 mL, no detrusor overactivity, and smooth
bladder contour with no vesico-ureteric reflux
(Fig 2). Bladder functional capacity was 200 mL.
It was therefore deemed worthwhile for him to
undergo renal transplantation without augmentation
cystoplasty.
The patient received a cadaveric renal graft
from a 14-year-old donor. The operation was
uneventful and he was no longer dialysis-dependent.
At 8 weeks after transplantation, his creatinine level
was 124 μmol/L (eGFR 63 mL/min/1.73 m2), and
urine output about 2000 mL per day. At 12 weeks
after the transplantation, his creatinine level was
122 μmol/L (eGFR 65 mL/min/1.73 m2), and urine
output remained stable at about 2000 mL per day.
Daytime frequency ranged from once every 1
to 3 hours, with 100 to 300 mL of urine per void.
At 22 weeks after transplantation his creatinine
level was 117 μmol/L (eGFR 68 mL/min/1.73 m2).
Ultrasonography excluded graft kidney
hydronephrosis and hydroureter.
Discussion
This is the first reported case of ketamine-associated nephropathy successfully treated with renal
transplantation.
Ketamine cystitis was first reported in Hong
Kong by Chu et al1 in 2007. Since then, numerous
publications regarding its management have
emerged. Abstinence remains the cornerstone
of treatment as it results not only in improved
cystitis symptoms, but also bladder capacity and
compliance.2 3 Early upper tract protection is
paramount in patients with ketamine cystitis. Up to
16.8% of chronic ketamine abusers have unilateral or
bilateral hydronephrosis owing to ureteric strictures
or vesico-ureteric reflux.4 Strategies to protect the
upper tract include percutaneous nephrostomy
or long-term urethral catheterisation to keep the
bladder decompressed.5 Internal ureteric stents
may aggravate cystitis symptoms and hence may
not be tolerated.6 In our case, the patient refused
percutaneous nephrostomies for upper tract
protection, and missed the window of opportunity
to preserve his renal function before development of
end-stage renal failure and need for dialysis.
There are two important prerequisites for renal transplantation in ketamine-related end-stage
renal failure. The first is abstinence to ensure
that the graft kidney and ureter are not subject to
ketamine toxicity. Significant improvements in
bladder compliance and capacity may be achieved
only after at least 1 year of abstinence.1 3 7 Since
improvement may take several years to stabilise,
we suggest that consideration for transplantation
should be at least 1 year after stabilisation of bladder
function improvement and proven by serial negative
urine toxicology screening. The second prerequisite
is that bladder compliance and capacity are sufficient
to accommodate the volume of urine produced by
the graft kidney. A well-functioning graft kidney
with a poorly compliant bladder can be damaged by
vesico-ureteric reflux. Serial urodynamic studies to
document improvements in bladder capacity before
considering renal transplantation are mandatory.
Although there is no absolute cut-off value for
satisfactory bladder volume before transplantation,
persistent vesico-ureteric reflux that does not
resolve or downgrade with ketamine abstinence
would be an indication for augmentation cystoplasty
prior to transplantation. This will avoid debilitating
urinary frequency after transplantation or early graft
failure due to vesico-ureteric reflux. In our patient,
bladder compliance and capacity improved steadily
with prolonged abstinence, as documented by serial
cystometrograms. Renal transplantation without
augmentation cystoplasty was therefore an option.
Patients with unsatisfactory bladder compliance
and capacity should be counselled for augmentation
cystoplasty before undergoing transplantation. This
concept can be likened to the use of augmentation
cystoplasty prior to renal transplantation in patients
with high-pressure neurogenic bladder.8 Nonetheless
augmentation cystoplasty for patients with ketamine
cystitis is technically challenging owing to the fibrotic
bladder with transmural thickening. Furthermore,
the need for clean intermittent self-catheterisation
afterwards may be cumbersome for this young
patient group, especially if substantial ketamine-related
bladder pain remains.
Frequent follow-up after transplantation to
monitor renal function, functional bladder capacity
in the form of a bladder diary, and ultrasonography to
exclude graft hydronephrosis should be maintained.
A video cystometrogram to exclude vesico-ureteric
reflux is mandatory should graft function deteriorate.
Early and sustained abstinence as well as
advocation for early upper tract urinary diversion
are important factors in the prevention of ketamine-related
nephropathy. Clinicians should maintain
a low threshold of suspicion for ketamine abuse in
young patients who present with recurrent lower
urinary tract symptoms.9 A population-based survey
of lower urinary tract symptoms in Hong Kong
adolescents revealed that of those who reported lower urinary tract symptoms, 6.6% were substance abusers.10
Management of the symptoms of ketamine
cystitis should adopt a stepwise approach starting with
abstinence and analgesics; failing that, intravesical
instillation of hyaluronic acid, hydrodistension, and
eventually augmentation cystoplasty.3
Author contributions
All authors contributed to the concept or design of the study, acquisition of the data, analysis or interpretation of the
data, drafting of the manuscript, and critical revision of the
manuscript for important intellectual content. All authors
had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding/support
This case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of
Helsinki and provided informed consent for the treatment/
procedures and consent for publication.
References
1. Chu PS, Kwok SC, Lam KM, et al. ‘Street ketamine’–associated bladder dysfunction: a report of ten cases. Hong
Kong Med J 2007;13:311-3.
2. Cheung RY, Chan SS, Lee JH, Pang AW, Choy KW,
Chung TK. Urinary symptoms and impaired quality of life
in female ketamine users: persistence after cessation of use.
Hong Kong Med J 2011;17:267-73.
3. Hong YL, Yee CH, Tam YH, Wong JH, Lai PT, Ng CF. Management of complications of ketamine abuse: 10 years’
experience in Hong Kong. Hong Kong Med J 2018;24:175-81. Crossref
4. Yee CH, Teoh JY, Lai PT, et al. The risk of upper urinary
tract involvement in patients with ketamine-associated
uropathy. Int Neurourol J 2017;21:128-32. Crossref
5. Chu P, Ma WK, Wong S, et al. The destruction of the lower urinary tract by ketamine abuse: a new syndrome? BJU Int
2008;102:1616-22. Crossref
6. Tsai YC, Kuo HC. Ketamine cystitis: Its urological impact and management. Urol Sci 2015;26:153-7. Crossref
7. Yee CH, Lai PT, Lee WM, Tam YH, Ng CF. Clinical outcome of a prospective case series of patients with
ketamine cystitis who underwent standardized treatment
protocol. Urology 2015;86:236-43. Crossref
8. Basiri A, Otookesh H, Hosseini R, Moghaddam SM. Kidney transplantation before or after augmentation cystoplasty in
children with high-pressure neurogenic bladder. BJU Int
2009;103:86-8. Crossref
9. Ng SH, Tse ML, Ng HW, Lau FL. Emergency department presentation of ketamine abusers in Hong Kong: a review
of 233 cases. Hong Kong Med J 2010;16:6-11.
10. Tam YH, Ng CF, Wong YS, et al. Population-based survey
of the prevalence of lower urinary tract symptoms in
adolescents with and without psychotropic substance
abuse. Hong Kong Med J 2016;22:454-63. Crossref

