Hong Kong Med J 2021 Jun;27(3):198–209 | Epub 30 Oct 2020
Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Incidence, patterns, risk factors, and
histopathological findings of liver injury in
coronavirus disease 2019 (COVID-19):
a scoping review
Taha Bin Arif, MB, BS; Saad Khalid, MB, BS; Mishal S Siddiqui, MB, BS; Harmla Hussain, MB, BS; Hassan Sohail, MB, BS
Department of Internal Medicine, Dow University of Health Sciences, Karachi, Pakistan
Corresponding author: Dr Taha Bin Arif (tahaarif20@yahoo.com)
Abstract
Background: Coronavirus disease 2019 (COVID-19)
exhibits many extrapulmonary manifestations,
including liver injury. This scoping review aimed
to provide insight into the incidence, patterns, risk
factors, histopathological findings, and relationship
with disease severity of COVID-19-associated liver
injury. Furthermore, we identified existing gaps in the
research on the hepatic manifestations of COVID-19
and highlighted areas for future investigations.
Methods: A scoping review was conducted following
the methodological framework suggested by Arksey
and O’Mallay. Five online databases, along with
grey literature, were searched for articles published
until 22 May 2020, and we included 62 articles in
the review. The research domains, methodological
characteristics, and key conclusions were included
in the analysis.
Results: Retrospective observational studies
comprised more than one-third (41.9%) of the
included publications, and 77.8% were conducted on
living patients. The incidence of liver injury varied
widely across the studies (4.8%-78%), and liver injury
was frequently associated with severe COVID-19.
We identified the following risk factors for liver
injury: male sex, lymphopoenia, gastrointestinal
involvement, old age, increased neutrophil count, and the use of hepatotoxic drugs. Histopathological
findings indicate that COVID-19 has direct
cytopathic effects and causes liver function test derangements
secondary to inflammation, hypoxia, and vascular
insult.
Conclusions: Liver injury following COVID-19
infection is common and primarily hepatocellular,
with a greater elevation of aspartate aminotransferase
tahn of alanine aminotransferase. However,
the evidence regarding hepatic failure secondary to
COVID-19 is insufficient. Standardised criteria
to diagnose liver injury need to be devised.
Current use of hepatotoxic drugs necessitates close
monitoring of liver function.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic
has spread to 213 countries and territories, posing
a severe threat to public healthcare systems
worldwide. As of 29 May 2020, the total number
of confirmed cases has surged to 5 701 337, with
357 688 deaths recorded worldwide.1 Although
multiple pharmacological agents are being evaluated,
no beneficial, targeted drug or vaccine has been
discovered to date, and the number of cases is rising
daily. The causative agent of COVID-19 is severe acute
respiratory syndrome coronavirus 2 (SARS-CoV-2),
which is believed to be transmitted through
respiratory droplets and person-to-person contact.
However, evidence of viral RNA in the faeces of
COVID-19 patients also suggests the possibility of
faecal-oral transmission.2 3 The disease typically presents with viral pneumonia-like symptoms of
fever, dry cough, shortness of breath, and fatigue.
Nevertheless, gastrointestinal symptoms like
diarrhoea, vomiting, and abdominal pain have also
been reported.4
Although coronavirus mainly targets
the respiratory system, it also exhibits many
extrapulmonary manifestations. Sepsis, acute
cardiac injury, multiple organ failure, and alkalosis
are some of the critical complications that have been
observed in patients who die of COVID-19.5 Several
studies have acknowledged the presence of liver
injury in patients with COVID-19, mainly indicated
by abnormal liver function tests (LFTs).6 7 The exact
pathophysiology behind the LFT derangements is
unknown. It has been suggested that SARS-CoV-2
causes liver injury either via direct viral insult or through an inflammatory cytokine storm.8 Other
potential mechanisms, such as drug-induced
hepatotoxicity and hypoxic injury, have also been
implicated.
Given the rampant nature of SARS-CoV-2
and its repercussions on human health, the research
community has responded expeditiously to the new
virus, and studies regarding its systemic involvement
are continuously being published. We have conducted
a scoping review to summarise all articles published
regarding hepatic damage in this setting. In this
review, we aim to provide evidence of the incidence,
patterns, risk factors, and histopathological findings
of liver injury in COVID-19 and its association with
the severity of disease. Furthermore, we highlight
hepatotoxicity in patients with COVID-19 who
are treated with antiviral (lopinavir/ritonavir)
or antimalarial (hydroxychloroquine) drugs. We
identify the existing gaps in current knowledge
regarding the topic and provide recommendations
for further research. This will help healthcare
providers to identify hepatic complications during
the pandemic.
Methods
Study design
A scoping review was conducted following the
methodological framework of Arksey and O’Malley9
by taking the following steps: (a) identification of a
definite research objective and search strategy; (b)
identification and screening of research articles;
(c) selection of research articles according to pre-defined eligibility criteria; (d) extraction and
charting of data, and (e) reporting, summarising,
and discussing the results.
Literature search strategies
The reviewed literature was identified by searching
five online databases (PubMed, Google Scholar,
Scopus, Wiley, and ScienceDirect) without any
language restriction from 1 January 2020 to
22 May 2020. Grey literature was also searched in
medRxiv and bioRxiv. Moreover, the reference lists
of all identified articles were searched for additional
sources. A variety of keywords were employed,
according to the following search string: “liver
injury” OR “hepatic damage” OR “liver functional
abnormality” OR “cirrhosis” OR “decompensated
liver disease” OR “acute liver failure” OR “chronic
liver failure” OR “acute on chronic liver failure” AND
“COVID-19” OR “SARS-CoV-2” OR “coronavirus
disease”. The full electronic search strategy is
provided in the online supplementary Appendix.
Identification, screening, and selection of
relevant studies
We aimed to summarise all of the scientific literature demonstrating liver dysfunction in COVID-19 and
to identify the gaps in knowledge regarding hepatic
damage in SARS-CoV-2 infection for further
research. Three researchers (TBA, SK, and MSS)
independently searched through the literature,
and all sets of literature were then compared.
Disagreements on the inclusion or exclusion of
literature were resolved through discussion or, if
necessary, by including a fourth researcher (HH)
to make the final decision. Articles were screened
according to pre-defined eligibility criteria. The
inclusion criteria were as follows: (1) study design:
retrospective observational study, retrospective
cohort study, retrospective descriptive study,
prospective observational study, prospective
case-cohort, cross-sectional, case report, case series,
or meta-analysis; (2) language: studies published in
English only; (3) publication status: preprints and
published articles; (4) dates considered: studies
published from 1 January 2020 to 22 May 2020;
and (5) all relevant papers describing functional
abnormalities of the liver in COVID-19. The
exclusion criteria were as follows: (1) language:
articles published in any language other than English; (2) study design: review article, editorial, letter to
the editor, correspondence, or commentary; and
(3) studies conducted on patients who had undergone
organ transplants. Duplicate articles were excluded.
Ultimately, 62 articles were included in this review
conducted in accordance with the Preferred
Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews (Fig 1).10

Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the scoping review process
Data extraction and charting from included
studies
After article selection, data were extracted and
recorded on a pre-designed datasheet. The extracted
data included the article’s title, study design, study
setting, study population, sample size, research
domain, and key conclusions.
Summarising the studies
The articles that assessed liver injury in patients
with COVID-19 belong to two categories: (a) studies
that employed pre-defined clinical criteria for
liver injury in COVID-19, and (b) studies that
did not employ any pre-defined criteria and only
reported LFT derangements in COVID-19. Based
on the primary research objectives, each article was
classified into one of the following main research
domains: incidence of liver injury, patterns of liver
injury, and risk factors for liver injury in COVID-19,
associations of liver injury or underlying liver
disease (eg, chronic liver disease) with the severity
of COVID-19, drug-induced liver injury in
COVID-19, and histopathological findings of
liver injury in COVID-19. The methodological
characteristics (study design, study setting, type of
population, and sample size) of all studies were also
analysed.
Results and discussion
Characteristics of studies
A total of 62 articles were included in this scoping
review, among which 10 were preprints, and 52
were published in peer-reviewed journals, including
The Lancet and Journal of the American Medical
Association. About 23 studies (16 retrospective
observational, 2 retrospective cohort, 1 retrospective
descriptive, 1 prospective observational, 1 prospective
case-cohort, 1 cross-sectional, and 1 meta-analysis)
documented the incidence of liver injury in
COVID-19.11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 Around half of the eligible studies
(n=29, 46.8%) showed an association between
the severity of COVID-19 and the degree of liver
injury.11 12 13 14 15 16 17 19 20 21 22 23 28 29 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48
Out of the 27 studies assessing liver injury in
COVID-19, 44.4% (n=12) had pre-defined clinical
criteria for liver injury, whereas 55.6% (n=15)
did not have any specific pre-defined criteria. The
details of these studies are given below. The studies predominantly depicted significant elevation of
aspartate aminotransferase (AST) than of alanine aminotransferase (ALT) in case of liver
injury, which was found to be proportional to the
severity of COVID-19.
Fewer studies (n=6) mentioned any
histopathological findings of liver injury in patients
with COVID-19, but the most common findings
mentioned were mild sinusoidal dilatation,
microvesicular steatosis, and minimal lymphocytic
infiltration.36 49 50 51 52 53 Eight studies assessed the impact
of drugs on potential liver damage. Half of those
studies (n=4, 50%) concluded that the use of
lopinavir/ritonavir increases the odds of liver injury.
Other drugs described as having the potential
to cause hepatotoxicity in COVID-19 included
hydroxychloroquine (n=1, 12.5%), tocilizumab (n=2,
25%), and remdesivir (n=1, 12.5%).12 18 54 55 56 57 58 59
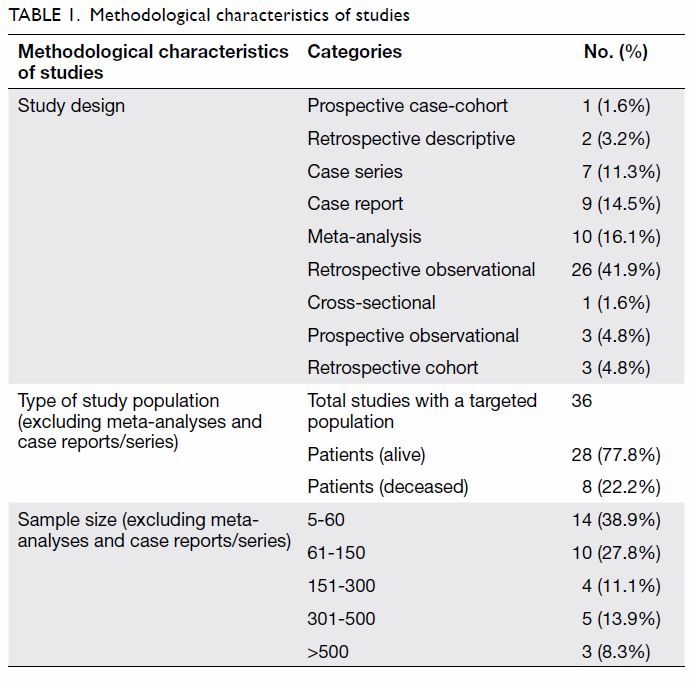
The methodological characteristics of
the finalised studies were also analysed. The
largest number of the studies were retrospective
observational studies (n=26, 41.9%), followed by
meta-analyses (n=10, 16.1%), case reports (n=9,
14.5%), case series (n=7, 11.3%), prospective
observational studies (n=3, 4.8%), and others (Table 1). All studies except for meta-analyses, case
reports, and series included a targeted population.
Among the 36 articles with a targeted population,
more than three-quarters (n=28, 77.8%) were
conducted only on living patients with COVID-19,
whereas the remainder (n=8, 22.2%) included
patients who died. Of the finalised studies, 38.9%
(n=14) had sample sizes of 5 to 60. The included
studies’ methodological characteristics are given in
Table 1.
Incidence of liver injury in patients with
COVID-19
Several observational studies documenting the
clinical characteristics of patients with COVID-19
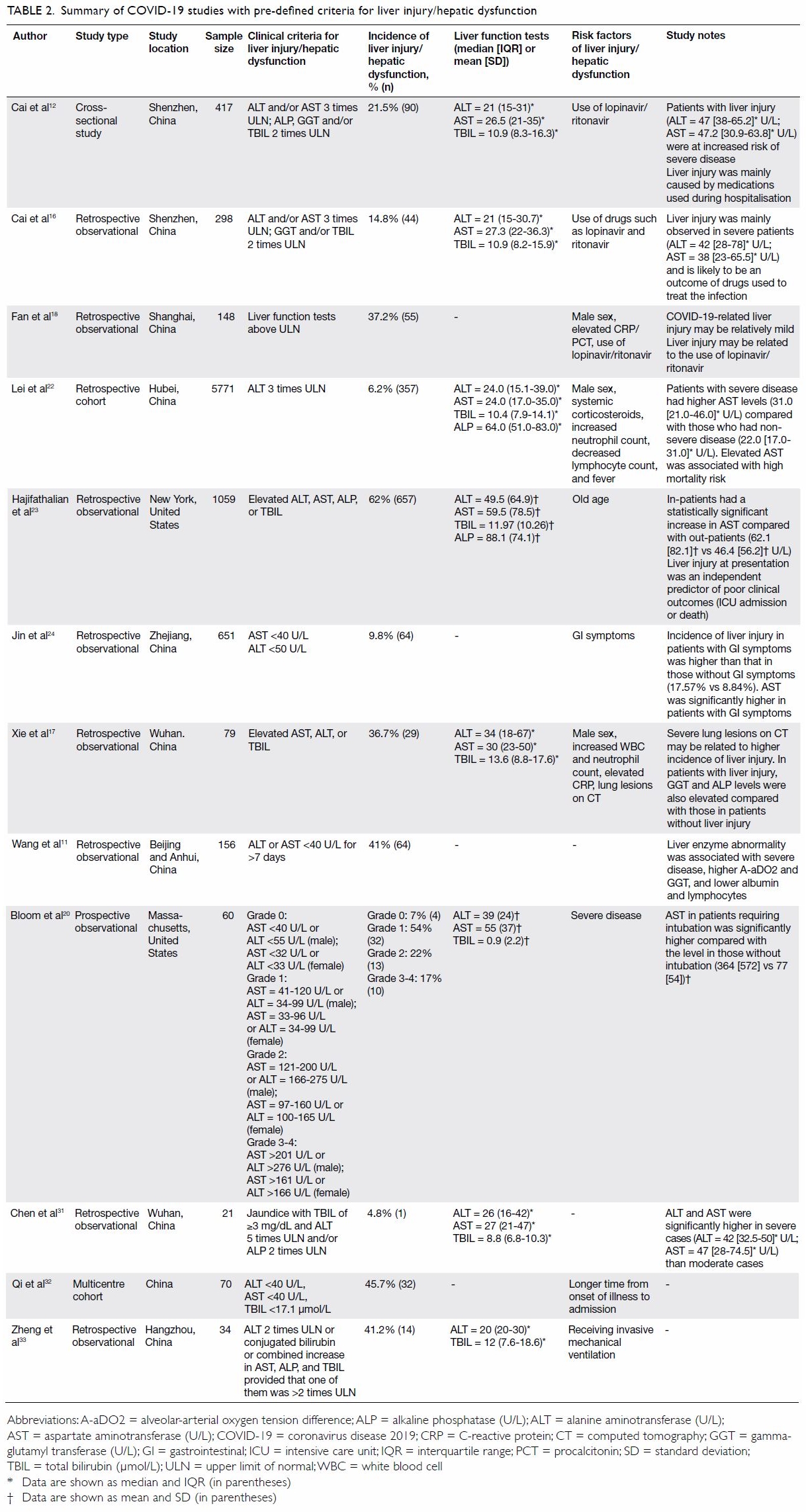
have reported liver injury.11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 They have mentioned
liver enzyme elevation without commenting on
the clinical signs of hepatic dysfunction, which
include hepatomegaly, ascites, and jaundice.11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 32
The incidence of liver injury has varied widely
across studies, from 4.8% to a striking 78%.11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33
However, the term ‘liver injury’ has not been
defined uniformly. The definitions used in various
studies have ranged from slight transaminasaemia
to enzyme elevation more than 3 times higher than
the upper limit of normal (Table 2 11 12 16 17 18 20 22 23 24 31 32 33).
Additionally, several studies did not establish any
clinical or laboratory criteria to define liver injury
(Table 3 13 14 15 19 21 25 26 27 28 29 34 35 37 46 60). Many studies failed to
mention the date on which LFTs were performed, thus creating non-uniformity in their reported
values. Case reports have identified the presence of
liver injury across the entire age spectrum, ranging
from 55 days to 65 years.50 57 58 61 62
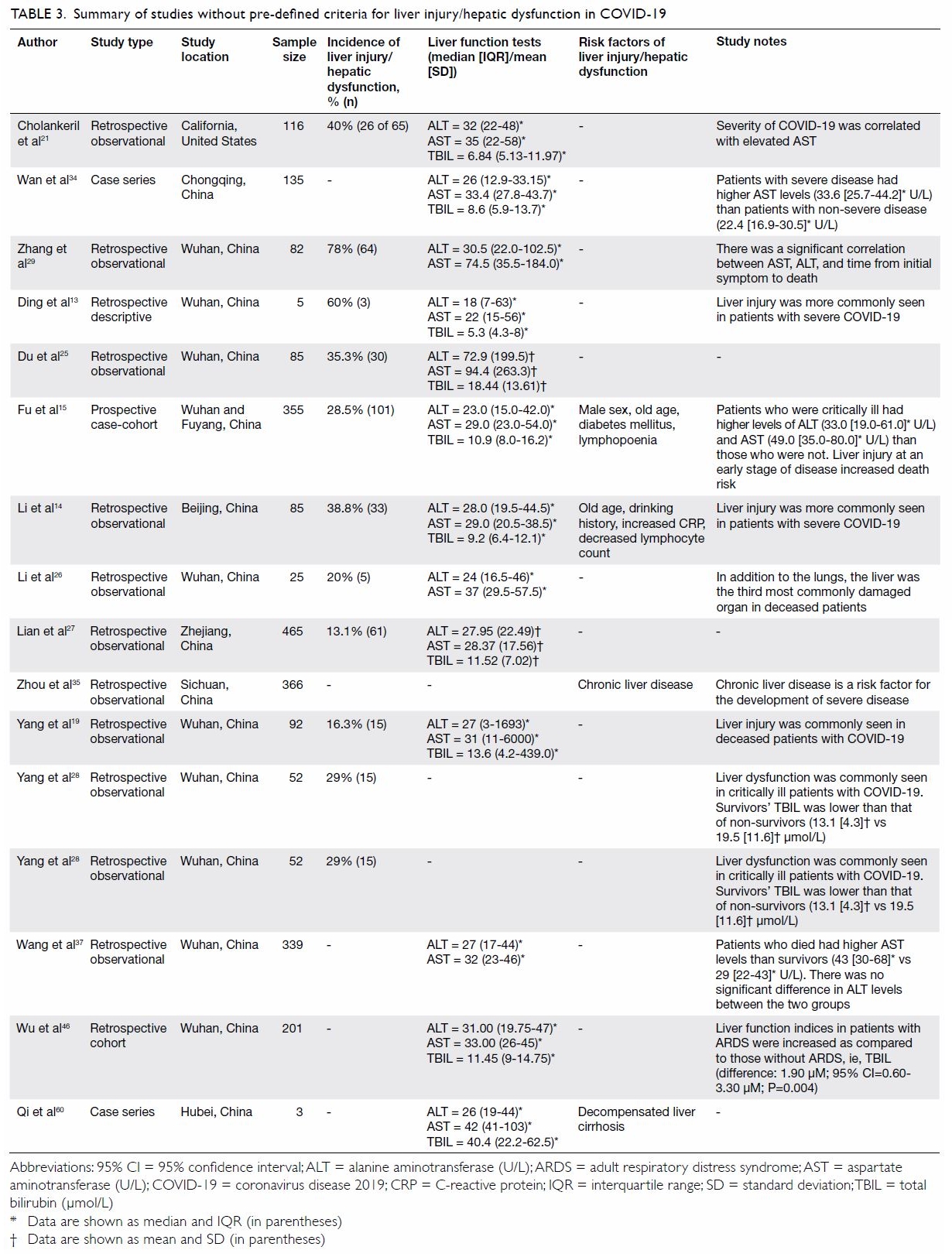
Table 3. Summary of studies without pre-defined criteria for liver injury/hepatic dysfunction in COVID-19
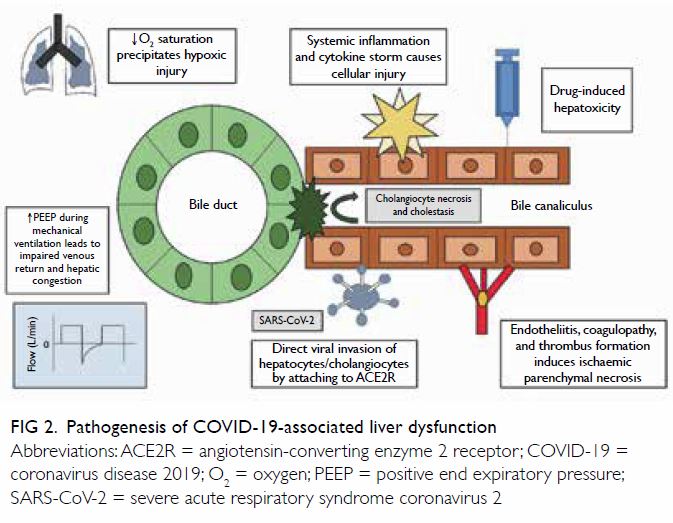
Pathogenesis of liver dysfunction in COVID-19
The pathogenesis of liver involvement in COVID-19 infection is assumed to be multifactorial. However,
none of the available hypotheses provide a complete
explanation, and further investigation is required
not only to understand the mechanism but also to
formulate appropriate management plans. Figure 2
illustrates the possible mechanisms of hepatic
dysfunction in COVID-19.
Direct viral invasion
The proposed receptor for the virus, angiotensinconverting
enzyme 2 receptor (ACE2R), has
been found only sparsely in hepatocytes. Chai
et al63 demonstrated ACE2 expression in 2.6% of
hepatocytes, whereas up to 59.7% of cholangiocytes
expressed ACE2R. Seow et al64 also revealed the
presence of ACE2 in liver progenitor cells, especially
those destined to become cholangiocytes. These
findings imply direct invasion of cholangiocytes
and progenitor cells, thus resulting in necrosis and
impaired regeneration of cholangiocytes. The tight
junctions between cholangiocytes also seem to be
altered during COVID-19 infection, which may be
responsible for the observed cholestasis in patients.65
Significant necrosis of and rapid viral replication
within cholangiocytes has also been observed by
Zhao et al65 in a human liver ductal organoid model.
However, Zhou et al66 argued against this
proposed mechanism by highlighting that ACE2Rs
on cholangiocytes are confined to the apical surface,
from where viral invasion is unlikely. Furthermore,
the hepatic pattern of LFT elevation fails to explain
this possible ductal pathology. Hepatocytes also
express the protein furin, which may play a role in
liver damage upon entry of the virus into the cells.
Hypoxia
Decreased oxygen saturation has been a feature
of COVID-19 pneumonia, and this may result in
hypoxic injury to multiple organs, including the
liver.67
High positive end expiratory pressure
High values of positive end expiratory pressure used
during mechanical ventilation in severe patients
may result in hepatic congestion by increasing the
pressure on the right atrium and thereby impeding
venous return. However, the presence of comparable
liver functional abnormalities in patients without
ventilation renders that assumption inconclusive.68
Systemic inflammation and cytokine storm
An inflammatory response to the virus may lead to
persistent leukocytic activation and the release of
many mediators responsible for cellular injury. The
involvement of a cytokine storm in liver damage
has been supported by patients’ elevated levels of
interleukins 2, 6, and 10, interferon-gamma, serum
ferritin, and C-reactive protein.17
Endothelial dysfunction
Liver dysfunction may occur secondary to vascular pathology, resulting in endotheliitis, coagulopathy,
thrombus formation, and ischaemic parenchymal
necrosis. Angiotensin-converting enzyme 2
receptors are expressed on the endothelium, making
it susceptible to viral invasion, which leads to the
recruitment of inflammatory cells and elaboration of
inflammatory cytokines. The immune response may
also exacerbate the damage.69
Drug-induced hepatotoxicity
A number of drugs currently in use have hepatotoxic potential, which might be further exaggerated in
the setting of chronic liver disease (CLD).12 56 58 The
mechanisms for individual drugs are not clearly
defined.
Patterns of liver injury in COVID-19
The patterns of liver injury in COVID-19 patients
include both reversible dysfunction and irreversible
injury as a component of multiorgan failure in
terminally ill patients.40 57 58 59 61 62 However, hepatic
dysfunction in COVID-19 cases is usually mild, and
deranged LFTs tend to recover within a few days after
discharge.48 Predominant elevation of ALT and AST
indicates hepatocellular injury.12 The abnormalities
in AST have been more severe compared with those
of ALT.20 22 23 34 37 44 45 This finding is intriguing, as
ALT, being more liver-specific, is the enzyme that
is generally expected to be significantly elevated in
case of hepatocellular injury. However, a few studies
have hypothesised that AST elevation could be
secondary to viral-mediated direct liver damage.20 21
The mechanism behind predominant AST elevation
in the presence of viral aetiology remains unclear.
Elevation of the ductal enzymes gamma-glutamyl
transferase and alkaline phosphatase
(ALP) has been reported in some studies.22 23 70
Elevated ALP was also reported alongside elevated
AST and ALT in a case of acute hepatitis following
COVID-19 infection.61 Cardoso et al71 studied the
temporal patterns of liver enzyme levels in critically
ill patients and observed that a cholestatic pattern
emerged later in the course of illness. Most studies
have not mentioned the presence of any liver enzyme
abnormalities at the time of liver injury. Hence, the
extent of liver damage and the pattern of injury could
not be accurately assessed.
Histopathological findings of liver injury in
COVID-19
Histopathological findings of autopsied liver samples
have provided evidence of direct viral invasion and
changes secondary to hypoxia, sepsis, and pro-inflammatory
and pro-coagulant states. Wang et al11
revealed the presence of hepatic apoptosis, occasional
bi- or multi-nucleated hepatocytes, mitochondrial
swelling, and decreased glycogen granules. These
findings strongly suggest direct cytopathic effects
of COVID-19 on the liver. Electron microscopy
also showed the presence of viral particles. Other
non-specific findings have included varying
degrees of steatosis,11 12 49 50 51 52 mild portal lymphocytic
infiltration,11 36 50 51 mild sinusoidal dilation,36,51,53
and inflamed cells within the sinusoids.12 However,
samples obtained via needle biopsy did not facilitate
effective determination of the histology of the
ductal epithelium, which carries a higher density
of ACE2Rs.51 Ductal pathology was highlighted by Lax et al,52 indicating the presence of canalicular
cholestasis and mild nuclear pleomorphism of
cholangiocytes. Patterns of both massive and focal
patchy necrosis were reported in the periportal
and centrilobular areas.51 52 The authors suggested
that sepsis and systemic inflammation might be
responsible for acute hepatic necrosis. Furthermore,
reverse transcription-polymerase chain reaction
of one liver sample was positive for COVID-19.51
In that case, an ultrasound-guided autopsy
observed centrilobular congestion (which was likely
attributable to shock), ischaemic necrosis, portal
tract inflammation, and Kupffer cell activation.51
The watery degeneration of some hepatocytes
observed by Cai et al12 was likely due to ischaemia
and hypoxia. The presence of thrombi within the
liver, among other organs, also demonstrates the
possibility of COVID-associated coagulopathy.52
Liver involvement with COVID-19 infection may
further elaborate the inflammatory cascade and
alter the secretion of coagulation factors, thus
playing a role in causing widespread thrombosis.52
Endotheliitis, acute and chronic vascular changes,
and sinusoidal arterialisation due to pressure
elevation observed in the liver further support the
involvement of underlying endothelial pathology in
causing coagulative derangements.53 69
Liver injury as a marker of the severity of
COVID-19
Studies have consistently shown liver injury to
be associated with severe COVID-19.11 12 13 14 15 16 34 42 43 44
Deranged LFTs have also been linked to prolonged
hospital stays18 and worse clinical outcomes.19 38 40 45
Disease severity is most likely linked with the
elevation of AST rather than ALT.20 21 45 Additionally,
hypoproteinaemia and cholestasis in early-stage
disease have been shown to increase the risk of
death.15 However, the impact of AST on mortality
has been controversial.22 46
These findings cannot reliably establish that
elevated LFT levels were solely caused by COVID-19
infection, as many studies did not exclude patients
with CLD, nor did they consider other possible
reasons for liver enzyme elevation. Furthermore,
there is still not enough evidence to suggest
that mild derangement has a high likelihood of
progressing into fulminant liver failure. Yet, patients
with deranged LFT patterns of the hepatocellular
or mixed types at the time of admission or during
hospitalisation were more likely to progress to
severe disease,12 47 thus necessitating adequate
monitoring.38 44 45 48 Additionally, there is no evidence
that liver dysfunction can directly cause mortality in
patients with COVID-19.
Risk factors for liver injury in COVID-19
Studies have reported associations between multiple risk factors and liver injury in the setting of
SARS-CoV-2 infection:
1. Abnormal white blood cell parameters, including
elevated neutrophils and decreased lymphocytes,
have been associated with elevated risk of liver
injury.14 15 17 22 The loss of lymphocytes responsible
for suppression of the immune response during
viral infection may have contributed to the
damage.14 Similarly, high levels of C-reactive
protein and procalcitonin were associated with
increased risk of liver damage.14 17 18 The cytokine
storm and systemic inflammation might be
implicated, as they result in leukocyte activation
and the release of a large quantity of inflammatory
mediators that directly or indirectly damage
cells.14
2. The use of hepatotoxic drugs, including antivirals, hydroxychloroquine, tocilizumab (discussed below),12 16 18 58 and antifungals for superimposed infections has been established as a risk factor for liver dysfunction.22 Systemic corticosteroids were also associated with an increased risk of AST elevation,22 perhaps due to drug-induced lymphopoenia and alteration of the immune response.
3. A correlation between the severity of lung involvement and the incidence of liver injury has also been noticed.17 As severe lung lesions indicate a robust inflammatory state, the liver might be affected for the same reason (ie, a hyperinflammatory state).17 That study did not indicate the role of hypoxia, which is also a possible contributing factor to hepatic damage.
4. Non-modifiable risk factors such as male sex (odds ratio=1.60; P<0.001) and old age (odds ratio=1.01; P=0.031) have been linked to a higher risk of liver damage.22 23
5. Patients with elevated ALT levels were more likely to have a history of drinking (P=0.032).14 However, that study did not comment on elevation of AST, the dominant enzyme involved in both alcoholic liver disease and COVID-19. Furthermore, that study’s very small sample size necessitates further investigation of this risk factor.
6. Patients with gastrointestinal symptoms were more likely to have liver injury than those without such symptoms (P=0.035).24
7. Diabetes mellitus was a risk factor for cholestasis in patients with COVID-19 (P=0.044),15 which is predicted to be a possible mechanism of liver injury in the setting of viral infection.65
8. Invasive mechanical ventilation increased the risk of LFT elevation42 and acute liver injury.33 Such injury may be caused by hepatic congestion that results from elevated right atrial pressure, which in turn is caused by high levels of positive end expiratory pressure.68
2. The use of hepatotoxic drugs, including antivirals, hydroxychloroquine, tocilizumab (discussed below),12 16 18 58 and antifungals for superimposed infections has been established as a risk factor for liver dysfunction.22 Systemic corticosteroids were also associated with an increased risk of AST elevation,22 perhaps due to drug-induced lymphopoenia and alteration of the immune response.
3. A correlation between the severity of lung involvement and the incidence of liver injury has also been noticed.17 As severe lung lesions indicate a robust inflammatory state, the liver might be affected for the same reason (ie, a hyperinflammatory state).17 That study did not indicate the role of hypoxia, which is also a possible contributing factor to hepatic damage.
4. Non-modifiable risk factors such as male sex (odds ratio=1.60; P<0.001) and old age (odds ratio=1.01; P=0.031) have been linked to a higher risk of liver damage.22 23
5. Patients with elevated ALT levels were more likely to have a history of drinking (P=0.032).14 However, that study did not comment on elevation of AST, the dominant enzyme involved in both alcoholic liver disease and COVID-19. Furthermore, that study’s very small sample size necessitates further investigation of this risk factor.
6. Patients with gastrointestinal symptoms were more likely to have liver injury than those without such symptoms (P=0.035).24
7. Diabetes mellitus was a risk factor for cholestasis in patients with COVID-19 (P=0.044),15 which is predicted to be a possible mechanism of liver injury in the setting of viral infection.65
8. Invasive mechanical ventilation increased the risk of LFT elevation42 and acute liver injury.33 Such injury may be caused by hepatic congestion that results from elevated right atrial pressure, which in turn is caused by high levels of positive end expiratory pressure.68
Drug-induced hepatotoxicity in COVID-19
The drugs currently used to manage COVID-19
infection also carry hepatotoxic potential. Muhović
et al56 reported a 40-fold rise in transaminases
following two doses of tocilizumab, an interleukin-6
receptor antagonist, which regressed 10 days later.
Morena et al59 also reported elevated liver enzymes
in 29% of patients who were receiving tocilizumab.
In addition, Falcão et al58 reported a 10-fold
elevation in transaminases following two doses of
hydroxychloroquine. Upon withdrawal, the enzyme
levels dropped to near normal after 5 days.
Antivirals have also been demonstrated to
cause liver toxicity. In one study, people receiving
lopinavir/ritonavir had a higher incidence of liver
dysfunction compared with those in whom these
drugs were not administered (51.8% vs 31.3%,
respectively).18 Similarly, Young et al55 reported
abnormal LFTs in three out of five patients receiving
lopinavir/ritonavir. According to Cai et al,12 the use
of lopinavir/ritonavir increased the likelihood of
liver injury 4-fold. Durante-Mangoni et al54 reported
that remdesivir caused elevation of liver enzymes in
three out of four patients, and Weber et al57 suggested
that drugs may play a role in precipitating acute liver
failure. Administration of lopinavir/ritonavir and
interferon was followed by progressive worsening of
LFTs. This effect may have been attenuated by the use
of Ramipril for arterial hypertension.57 Additionally,
Lei et al22 showed that elevated AST and ALP
levels were associated with the use of antifungal
medications. The above findings are from case
reports, retrospective studies, and very small-scale
prospective studies. Further large-scale prospective
studies, including randomised controlled trials, need
to be conducted to establish their efficacy and safety
in patients with COVID-19.
Chronic liver disease and COVID-19
The effects of underlying liver disease on the
severity of COVID-19 are controversial. Zhou et al35
suggested the presence of CLD as a risk factor for
severe COVID-19. However, that study included
only eight known cases of CLD. Similarly, Qi et al60
indicated that decompensated liver cirrhosis might
be a risk factor for poor outcomes of COVID-19.
In contrast, a meta-analysis by Wang et al72 that
included five studies concluded that prior liver
disease does not impact the severity of COVID-19.
Likewise, the presence of pre-existing cirrhosis had
no direct prognostic association in the setting of
COVID-19.73 Some studies in our review included
patients with CLD, which may account for some
of the LFT derangements observed in patients. A
meta-analysis by Mantovani et al41 estimated that
the baseline prevalence of CLD was 3%. This figure is
much lower than the proportion of people with liver dysfunction. Hence, the role of CLD in worsening
the prognosis of COVID-19 infection seems to be
minor, if there is any.
Nevertheless, acute-on-chronic liver failure
(ACLF) following COVID-19 infection has been
reported. One case was a female patient with
decompensated alcoholic cirrhosis (ACLF Grade 2)
who developed a mixed hepatic and cholestatic
pattern of liver dysfunction following COVID-19
infection. However, her prognosis was good.74
Another patient was an older man with ACLF
Grade 1 non-alcoholic cirrhosis. He developed
hepatorenal syndrome-type acute kidney injury
following COVID-19 infection. His liver failure
subsequently progressed to Grade 2 after catheter-associated
urinary tract infections and complicated
paracentesis.75
Oro-faecal transmission and liver injury
Cui et al61 revealed that anal swabs of an infant
with liver injury remained positive for COVID-19
even after throat swabs returned to a negative state.
However, polymerase chain reaction of stool samples
was not performed in most of the articles included
in our review. The association of liver dysfunction
with the risk of oro-faecal transmission remains to
be investigated. If transmission via this route is
possible, existing isolation and discharge protocols
may need to be revised.
Limitations and recommendations
Our review is subject to certain limitations. First,
the majority of the included studies did not have any
specific pre-defined clinical criteria for diagnosing
liver injury in COVID-19. Moreover, the included
studies did not distinguish between a history of
liver disease (eg, CLD) and liver injury secondary to
COVID-19. Hence, our results need to be interpreted
cautiously, as they do not accurately describe the
level of incidence of liver injury that is caused by
COVID-19. Second, we did not include studies
published in any language other than English, which
might have provided additional insight. Third, the
inclusion of a large number of studies prevented
us from critically appraising the individual
studies’ sources of evidence. There is a need for a
comprehensive systematic review or meta-analysis
to summarise the statistics and provide a clearer
picture of liver injury in SARS-CoV-2 infection.
Transplant recipients, a group that is vulnerable to
liver injury, were also not reviewed.
Many studies have defined ‘liver injury’ as
non-specific elevation of LFTs above the upper limit
of normal. Further, many of the investigated studies
did not assess the bilirubin levels or coagulation
profiles of patients with COVID-19, both of which
are important indicators of liver function. Moreover, there have been no reports of liver failure or hepatic
cell death secondary to COVID-19 to date. Hence, we
recommend the use of scientifically relevant terms
‘liver dysfunction’ or ‘liver enzyme derangement’
to explain non-specific LFT abnormalities until an
appropriate definition for liver injury is devised.
Furthermore, we propose that pre-defined criteria for
liver injury should be set and that mild, non-specific
derangements of liver function should not be labelled
as liver injury. Given the existing controversy in the
literature, we recommend a thorough investigation
into the pathogenesis of liver injury, especially the
mode of direct viral invasion.
Additional studies are required to investigate
whether mild derangement of liver function can
cause hepatic failure in COVID-19. The reason for
the hepatocellular pattern with predominant AST
elevation also needs to be elucidated. Finally, the
safety and efficacy of hepatotoxic drugs in COVID-19
should also be established via randomised controlled
trials.
Conclusion
Liver injury is a common extrapulmonary feature of
COVID-19. However, the absence of standardised
clinical criteria for liver injury in this setting needs
to be addressed. Derangements of LFT levels are
markers of the severity of COVID-19 infection, but
the association between LFT derangements and
disease progression requires further investigation
because to date, liver dysfunction has not been
shown to directly cause mortality in patients with
COVID-19. The pattern of injury is predominantly
hepatocellular, accompanied by greater elevation
of AST than of ALT. Possible pathogenetic
mechanisms include direct viral invasion, hypoxia,
systemic inflammation, endothelial dysfunction, and
the use of mechanical ventilation. Histopathological
findings in the liver support viral-induced pathology
in addition to non-specific changes. Nevertheless,
these studies are sparse, and more research is
required. Potentially hepatotoxic drugs have also
been observed to cause liver injury in patients with
COVID-19, and thus, the administration of these
drugs necessitates careful monitoring. Large-scale
studies are needed to establish their role in the
management of COVID-19.
Author contributions
Concept or design: T Bin Arif.
Acquisition of data: T Bin Arif, S Khalid, MS Siddiqui, H Hussain.
Analysis or interpretation of data: H Sohail.
Drafting of the manuscript: S Khalid, MS Siddiqui, H Hussain.
\ Critical revision of the manuscript for important intellectual content: T Bin Arif, H Sohail.
Acquisition of data: T Bin Arif, S Khalid, MS Siddiqui, H Hussain.
Analysis or interpretation of data: H Sohail.
Drafting of the manuscript: S Khalid, MS Siddiqui, H Hussain.
\ Critical revision of the manuscript for important intellectual content: T Bin Arif, H Sohail.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
1. World Health Organization. Coronavirus disease
(COVID-2019) situation reports–124. Available from:
https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200523-covid-19-sitrep-124.pdf?sfvrsn=9626d639_2. Accessed 29 May 2020.
2. Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020;323:1843-
4. Crossref
3. Chen J, Zhu H, Wang D, et al. Clinical features of stool
SARS-CoV-2 RNA positive in 137 COVID-19 patients in
Taizhou, China. SSRN Electronic J 2020 Mar 23. Available
from: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3551383. Accessed 28 May 2020. Crossref
4. Pan L, Mu M, Yang P, et al. Clinical characteristics of
COVID-19 patients with digestive symptoms in Hubei,
China: a descriptive, cross-sectional, multicenter study.
Am J Gastroenterol 2020;115:766-73. Crossref
5. Chen T, Wu D, Chen H, et al. Clinical characteristics of
113 deceased patients with coronavirus disease 2019:
retrospective study. BMJ 2020;368:m1091. Crossref
6. Chen N, Zhou M, Dong X, et al. Epidemiological and
clinical characteristics of 99 cases of 2019 novel coronavirus
pneumonia in Wuhan, China: a descriptive study. Lancet
2020;395:507-13. Crossref
7. Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol
Hepatol 2020;5:428-30. Crossref
8. Mehta P, McAuley DF, Brown M, et al. COVID-19: consider
cytokine storm syndromes and immunosuppression.
Lancet 2020;395:1033-4. Crossref
9. Arksey H, O’Malley L. Scoping studies: towards a
methodological framework. Int J Soc Res Methodol
2005;8:19-32. Crossref
10. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for
scoping reviews (PRISMA-ScR): checklist and explanation.
Ann Inter Med 2018;169:467-73. Crossref
11. Wang Y, Liu S, Liu H, et al. SARS-CoV-2 infection of the
liver directly contributes to hepatic impairment in patients
with COVID-19. J Hepatol 2020;73:807-16. Crossref
12. Cai Q, Huang D, Yu H, et al. COVID-19: Abnormal liver
function tests. J Hepatol 2020;73:566-74. Crossref
13. Ding Q, Lu P, Fan Y, Xia Y, Liu M. The clinical characteristics
of pneumonia patients coinfected with 2019 novel
coronavirus and influenza virus in Wuhan, China. J Med
Virol 2020 Mar 20. Epub ahead of print. Crossref
14. Li L, Li S, Xu M, et al. Risk factors related to hepatic injury
in patients with corona virus disease 2019. medRxiv. 2020
Mar 10. Available from: https://www.medrxiv.org/content/10.1101/2020.02.28.20028514v2. Accessed 27 May 2020. Crossref
15. Fu L, Fei J, Xu S, et al. Acute liver injury and its association
with death risk of patients with COVID-19: a hospital-based prospective case-cohort study. medRxiv. 2020 Apr 6.
Available from: https://www.medrxiv.org/content/10.1101/
2020.04.02.20050997v1. Accessed 27 May 2020. Crossref
16. Cai Q, Huang D, Ou P, et al. COVID-19 in a designated
infectious diseases hospital outside Hubei Province, China.
Allergy 2020;75:1742-52. Crossref
17. Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical
characteristics of non-ICU hospitalized patients with
coronavirus disease 2019 and liver injury: a retrospective
study. Liver Int 2020;40:1321-6. Crossref
18. Fan Z, Chen L, Li J, et al. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol
Hepatol 2020;18:1561-6. Crossref
19. Yang F, Shi S, Zhu J, Shi J, Dai K, Chen X. Analysis of 92
deceased patients with COVID-19. J Med Virol 2020 Apr
15. Epub ahead of print. Crossref
20. Bloom PP, Meyerowitz EA, Reinus Z, et al. Liver biochemistries in hospitalized patients with COVID-19.
Hepatology 2020 May 16. Epub ahead of print. Crossref
21. Cholankeril G, Podboy A, Aivaliotis VI, et al. High
prevalence of concurrent gastrointestinal manifestations
in patients with SARS-CoV-2: early experience from
California. Gastroenterology 2020;159:775-7. Crossref
22. Lei F, Liu YM, Zhou F, et al. Longitudinal association
between markers of liver injury and mortality in COVID19
in China. Hepatology 2020;72:389-98. Crossref
23. Hajifathalian K, Krisko T, Mehta A, et al. Gastrointestinal
and hepatic manifestations of 2019 novel coronavirus
disease in a large cohort of infected patients from New York:
clinical implications. Gastroenterology 2020;159:1137-40.
e2. Crossref
24. Jin X, Lian JS, Hu JH, et al. Epidemiological, clinical and
virological characteristics of 74 cases of coronavirus-infected
disease 2019 (COVID-19) with gastrointestinal
symptoms. Gut 2020;69:1002-9. Crossref
25. Du Y, Tu L, Zhu P, et al. Clinical features of 85 fatal cases
of COVID-19 from Wuhan: a retrospective observational
study. Am J Respir Crit Care Med 2020;201:1372-9. Crossref
26. Li X, Wang L, Yan S, et al. Clinical characteristics of 25
death cases with COVID-19: a retrospective review of
medical records in a single medical center, Wuhan, China.
Int J Infect Dis 2020;94:128-32. Crossref
27. Lian J, Jin X, Hao S, et al. Epidemiological, clinical, and
virological characteristics of 465 hospitalized cases of
coronavirus disease 2019 (COVID-19) from Zhejiang
province in China. Influenza Other Respir Viruses
2020;14:564-74. Crossref
28. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes
of critically ill patients with SARS-CoV-2 pneumonia
in Wuhan, China: a single-centered, retrospective,
observational study. Lancet Respir Med 2020;8:475-81. Crossref
29. Zhang B, Zhou X, Qiu Y, et al. Clinical characteristics of
82 death cases with COVID-19. medRxiv. 2020 Feb 27.
Available from: https://www.medrxiv.org/content/10.1101/2020.02.26.20028191v1. Accessed 28 May 2020. Crossref
30. Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3062
COVID-19 patients: a meta-analysis. J Med Virol 2020 Apr
15. Epub ahead of print. Crossref
31. Chen G, Wu D, Guo W, et al. Clinical and immunological
features of severe and moderate coronavirus disease 2019.
J Clin Invest 2020;130:2620-9. Crossref
32. Qi X, Liu C, Jiang Z, et al. Multicenter analysis of clinical
characteristics and outcome in patients with COVID-19 who develop liver injury. J Hepatol 2020;73:455-8. Crossref
33. Zheng Y, Sun L, Xu M, et al. Clinical characteristics of 34
COVID-19 patients admitted to ICU in Hangzhou, China.
medRxiv. 2020 Apr 15. Available from: https://www.medrxiv.org/content/10.1101/2020.04.12.20062604v1.Accessed 28 May 2020. Crossref
34. Wan S, Xiang Y, Fang W, et al. Clinical features and
treatment of COVID-19 patients in Northeast Chongqing.
J Med Virol 2020;92:797-806. Crossref
35. Zhou Y, He Y, Yang H, et al. Development and validation
a nomogram for predicting the risk of severe COVID-19:
a multi-center study in Sichuan, China. PLoS One
2020;15:e0233328. Crossref
36. Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver
impairment in COVID-19 patients: a retrospective analysis
of 115 cases from a single centre in Wuhan City, China.
Liver Int 2020;40:2095-103. Crossref
37. Wang L, He W, Yu X, et al. Coronavirus disease 2019 in
elderly patients: characteristics and prognostic factors
based on 4-week follow-up. J Infect 2020;80:639-45. Crossref
38. Parohan M, Yaghoubi S, Seraj A. Liver injury is associated
with severe coronavirus disease 2019 (COVID-19)
infection: a systematic review and meta-analysis of
retrospective studies. Hepatol Res 2020;50:924-35. Crossref
39. Mao R, Qiu Y, He JS, et al. Manifestations and prognosis
of gastrointestinal and liver involvement in patients with
COVID-19: a systematic review and meta-analysis. Lancet
Gastroenterol Hepatol 2020;5:667-78. Crossref
40. Henry BM, de Oliveira MH, Benoit S, Plebani M, Lippi G.
Hematologic, biochemical and immune biomarker
abnormalities associated with severe illness and mortality
in coronavirus disease 2019 (COVID-19): a meta-analysis.
Clin Chem Lab Med 2020;58:1021-8. Crossref
41. Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease
2019 and prevalence of chronic liver disease: a meta-analysis.
Liver Int 2020;40:1316-20. Crossref
42. Israelsen SB, Kristiansen KT, Hindsberger B, et al.
Characteristics of patients with COVID-19 pneumonia
at Hvidovre Hospital, March-April 2020. Dan Med J
2020;67:A05200313.
43. Zhao X, Lei Z. The impact of coronavirus disease 2019
(COVID-19) on liver injury in China: a systematic review
and meta-analysis. medRxiv. 2020 May 8. Available from:
https://www.medrxiv.org/content/10.1101/2020.05.03.20089557v1. Accessed 28 May 2020. Crossref
44. Dong X, Zeng DY, Cai YY, et al. Liver chemistries in
patients with severe or non-severe COVID-19: a metaanalysis.
medRxiv. 2020 Apr 29. Available from: https://
www.medrxiv.org/content/10.1101/2020.04.24.20074179v1. Accessed 29 May 2020. Crossref
45. Xing QQ, Dong X, Ren YD, et al. Liver chemistries in
COVID-19 patients with survival or death: a metaanalysis.
medRxiv. 2020 May 1. Available from: https://www.medrxiv.org/content/10.1101/2020.04.26.20080580v1. Accessed 28 May 2020. Crossref
46. Wu C, Chen X, Cai Y, et al. Risk factors associated with
acute respiratory distress syndrome and death in patients
with coronavirus disease 2019 pneumonia in Wuhan,
China. JAMA Intern Med 2020;180:1-11. Crossref
47. Xu L, Mao Y, Chen G. Risk factors for severe corona virus
disease 2019 (COVID-19) patients: a systematic review
and meta analysis. medRxiv. 2020 Apr 1. Available from:
https://www.medrxiv.org/content/10.1101/2020.03.30.20047415v1. Accessed 28 May 2020. Crossref
48. Chen C, Jiang J, Xu X, Hu Y, Hu Y, Zhao Y. Dynamic liver
function indexes monitoring and clinical characteristics in
three types of COVID-19 patients. medRxiv. 2020 May 20.
Available from: https://www.medrxiv.org/content/10.1101/2020.05.13.20099614v2. Accessed 28 May 2020. Crossref
49. Nunes Duarte-Neto A, de Almeida Monteiro RA, da Silva LF,
et al. Pulmonary and systemic involvement of COVID-19
assessed by ultrasound-guided minimally invasive autopsy.
Histopathology 2020 May 22. Epub ahead of print. Crossref
50. Xu Z, Shi L, Wang Y, et al. Pathological findings of
COVID-19 associated with acute respiratory distress
syndrome. Lancet Respir Med 2020;8:420-2. Crossref
51. Tian S, Xiong Y, Liu H, et al. Pathological study of the
2019 novel coronavirus disease (COVID-19) through
postmortem core biopsies. Modern Pathol 2020;33:1007-14. Crossref
52. Lax SF, Skok K, Zechner P, et al. Pulmonary arterial
thrombosis in COVID-19 with fatal outcome: results from
a prospective, single-center, clinicopathologic case series.
Ann Intern Med 2020 May 14. Epub ahead of print. Crossref
53. Sonzogni A. Liver histopathology in COVID 19 infection
is suggestive of vascular alteration. 2020 May 11. Available
from: https://www.medrxiv.org/content/10.1101/2020.05.
06.20092718v1. Accessed 28 May 2020. Crossref
54. Durante-Mangoni E, Andini R, Bertolino L, et al. Early
experience with remdesivir in SARS-CoV-2 pneumonia.
Infection 2020;48:779-82. Crossref
55. Young BE, Ong SW, Kalimuddin S, et al. Epidemiologic
features and clinical course of patients infected with SARS-CoV-
2 in Singapore. JAMA 2020;323:1488-94. Crossref
56. Muhović D, Bojović J, Bulatović A, et al. First case of drug-induced
liver injury associated with the use of tocilizumab
in a patient with COVID-19. Liver Int 2020 May 17. Epub
ahead of print. Crossref
57. Weber S, Mayerle J, Irlbeck M, Gerbes AL. Severe liver
failure during SARS-CoV-2 infection. Gut 2020;69:1365-7. Crossref
58. Falcão MB, Pamplona de Góes Cavalcanti L, Filgueiras Filho
NM, Antunes de Brito CA. Case report: hepatotoxicity
associated with the use of hydroxychloroquine in a patient
with COVID-19. Am J Trop Med Hyg 2020;102:1214-6. Crossref
59. Morena V, Milazzo L, Oreni L, et al. Off-label use of
tocilizumab for the treatment of SARS-CoV-2 pneumonia
in Milan, Italy. Eur J Intern Med 2020;76:36-42. Crossref
60. Qi X, Wang J, Li X, et al. Clinical course of COVID-19 in
patients with pre-existing decompensated cirrhosis: initial
report from China. Hepatol Int 2020;14:478-82. Crossref
61. Wander P, Epstein M, Bernstein D. COVID-19 presenting
as acute hepatitis. Am J Gastroenterol 2020;115:941-2. Crossref
62. Cui Y, Tian M, Huang D, et al. A 55-day-old female infant
infected with 2019 novel coronavirus disease: presenting
with pneumonia, liver injury, and heart damage. J Infect
Dis 2020;221:1775-81. Crossref
63. Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in
cholangiocytes may cause liver damage after 2019-nCoV
infection. bioRxiv. 2020 Feb 4. Available from: https://www.biorxiv.org/content/10.1101/2020.02.03.931766v1. Accessed 28 May 2020. Crossref
64. Seow JJ, Pai R, Mishra A, et al. scRNA-seq reveals ACE2
and TMPRSS2 expression in TROP2+ liver progenitor cells:
implications in COVID-19 associated liver dysfunction.
bioRxiv. 2020 Mar 25. Available from: https://www.biorxiv.org/content/10.1101/2020.03.23.002832v1. Accessed 28 May 2020.
65. Zhao B, Ni C, Gao R, et al. Recapitulation of SARS-CoV-2
infection and cholangiocyte damage with human liver
ductal organoids. Protein Cell 2020;11:771-5. Crossref
66. Zhou L, Niu Z, Jiang X, et al. Systemic analysis of tissue
cells potentially vulnerable to SARS-CoV-2 infection by
the protein-proofed single-cell RNA profiling of ACE2,
TMPRSS2 and Furin proteases. 2020 Apr 18. Available
from: https://www.biorxiv.org/content/10.1101/2020.04.06.028522v2. Accessed 28 May 2020. Crossref
67. Waseem N, Chen PH. Hypoxic hepatitis: a review and
clinical update. J Clin Transl Hepatol 2016;4:263-8.
68. Kukla M, Skonieczna-Żydecka K, Kotfis K, et al. COVID-19,
MERS and SARS with concomitant liver injury—systematic
review of the existing literature. J Clin Med 2020;9:1420. Crossref
69. Varga Z, Flammer AJ, Steiger P, et al. Endothelial
cell infection and endotheliitis in COVID-19. Lancet
2020;395:1417-8. Crossref
70. Tian S, Zhu X, Sun X, et al. Longitudinal analysis of
laboratory findings during the process of recovery for patients with COVID-19. medRxiv. 2020 Apr 7. Available
from: https://www.medrxiv.org/content/10.1101/2020.04.04.20053280v1. Accessed 28 May 2020. Crossref
71. Cardoso FS, Pereira R, Germano N. Liver injury in
critically ill patients with COVID-19: a case series. Crit
Care 2020;24:190. Crossref
72. Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase
the risk of patients with COVID-19: evidence from meta-analysis.
Aging (Albany NY) 2020;12:6049-57. Crossref
73. Qi X, Liu Y, Wang J, et al. Clinical course and risk factors
for mortality of COVID-19 patients with pre-existing
cirrhosis: a multicentre cohort study. Gut 2020 May 20.
Epub ahead of print. Crossref
74. Qiu H, Wander P, Bernstein D, Satapathy SK. Acute on
chronic liver failure from novel severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2). Liver Int
2020;40:1590-3. Crossref
75. Große K, Kramer M, Trautwein C, Bruns T. SARS-CoV-2
as an extrahepatic precipitator of acute-on-chronic liver
failure. Liver Int 2020;40:1792-3. Crossref