© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Epidemiology and outcomes of geriatric and
non-geriatric patients with drug allergy labels in Hong Kong
Philip H Li, FHKCP, FHKAM (Medicine); HY Chung, FHKCP, FHKAM (Medicine); CS Lau, MD, FRCP
Division of Rheumatology and Clinical Immunology, Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Hong Kong
Corresponding author: Dr Philip H Li (liphilip@hku.hk)
Abstract
Introduction: Adverse drug reactions are more
common in geriatric patients than in younger
patients, but there have been insufficient studies
concerning the epidemiology or burden of drug
allergy labels in geriatric patients. We prospectively
investigated the prevalence and outcomes of
geriatric patients with drug allergy labels in a cohort
of hospitalised patients.
Methods: Patients admitted to a regional hospital
over a 6-month period were recruited for this
study. All patients with drug allergy labels were
prospectively followed until discharge; clinical data
were anonymously extracted for analyses. Patients
were categorised into either geriatric (aged ≥65
years) or non-geriatric (aged <65 years) groups.
Demographic characteristics, clinical outcomes, and
prevalences of drug allergy labels were compared
between groups.
Results: There were 4361 admissions involving
3641 patients during the 6-month study period.
Overall, 492 patients (13.5%) had drug allergy labels,
consisting of 151 non-geriatric patients (30.7%) and
341 geriatric patients (69.3%). The prevalence of drug
allergy labels did not significantly differ between
geriatric and non-geriatric patients (13.5% vs 13.5%, P=0.976). Significantly more patients in the geriatric
group had drug allergy labels to cardiovascular
system drugs (15.5% vs 4.6%, P=0.001). Geriatric
patients had a significantly lower rate of direct
discharge from the hospital (73.0% vs 88.1%,
P<0.001) and required transfers to convalescent or
rehabilitation care for further management.
Conclusions: More than 13% of hospitalised geriatric
patients had drug allergy labels. The leading causes
of drug allergy labels were similar between geriatric
and non-geriatric patients. Geriatric patients with
drug allergy labels had significantly more labelled
allergies to cardiovascular system drugs and adverse
clinical outcomes.
New knowledge added by this study
- More than 13% of hospitalised geriatric patients in Hong Kong had drug allergy (DA) labels.
- The most common DA labels were similar between geriatric and non-geriatric patients.
- Geriatric patients had significantly more DA labels to cardiovascular drugs and significantly lower direct discharge rates.
- Clinicians should consider the large burden of reported DAs and associated adverse clinical outcomes among hospitalised geriatric patients, particularly with respect to antibiotic therapy and cardiovascular system drugs.
- Geriatric patients with reported DAs should be selectively referred for formal allergy workup to confirm or refute suspected DAs.
Introduction
With the continued increase in life expectancy
worldwide, population ageing is an especially
marked phenomenon in Asian populations.1 It has been estimated that nearly one in three individuals
will be in the geriatric age-group (aged ≥65 years) in
Hong Kong within the next 15 years.2 Unfortunately,
improved longevity is not necessarily linked
with improved health or healthcare. Ageing is an unavoidable process associated with many age-related
diseases. For example, “immunosenescence”—the
age-related dysregulation of the immune system—increases geriatric patients’ susceptibilities to
a myriad of immune-mediated disorders (eg,
infection, malignancy, and autoimmunity) and
adverse reactions to medications.3 4
Adverse drug reactions (ADRs) are much more
common in geriatric patients, such that they cause significant morbidity and mortality, compared with
younger patients.5 6 Geriatric patients are much more
likely than younger patients to be hospitalised for
ADRs.7 In particular, drug allergies (DAs) comprise
approximately 6% to 10% of all ADRs and up to
10% of the resulting fatal reactions.6 Despite the
severe consequences of genuine DAs, many patients
mistakenly report non-immune-mediated ADRs as
“allergies”. For example, almost 90% of patients with
beta-lactam DA labels were confirmed not to be
genuinely allergic in previous studies, although such
incorrect DA labels were associated with a multitude
of dire clinical consequences.8 9 10 11 12 To the best of our
knowledge, although the prevalence of ADRs has
been extensively reported in geriatric populations,
there have been no adequate studies concerning
the epidemiology or burden of DAs in geriatric
patients.13 To address this lack of information, we
performed a prospective analysis of the prevalence
and outcomes of geriatric patients with DA labels in
a cohort of hospitalised patients in Hong Kong.
Methods
All patients admitted to the acute general medical
wards of Queen Mary Hospital from 1 July 2018 to
31 December 2018 were recruited for this study. The
Queen Mary Hospital is the only public hospital in the
Hong Kong West Cluster, which serves a population
of 0.5 million and provides acute medical admissions
through its Accident and Emergency Department.
After admission, patients may be transferred to
other convalescent or rehabilitation units of the
Hong Kong West Cluster for further management if
deemed unfit for direct discharge from the hospital.
Patient age and sex were recorded, as was the
presence of DA labels. All patients with DA labels
were then followed until discharge; clinical data
were anonymously extracted for analyses. Extracted
clinical data included patient age and sex, presence
and details of DA labels, length of stay (from the day
of admission to the day of discharge [including stay
at convalescent or rehabilitation hospitals] or death),
and discharge outcomes (direct discharge, transfer to
another hospital, or death). Details of DA labels were
reviewed to ensure that the reported manifestations
were consistent with the presence of clinical allergies
(ie, immune-mediated hypersensitivity reactions).
Manifestations suggestive of other non-immune-mediated
ADRs were excluded.
The DA labels were categorised in accordance
with the British National Formulary classifications (if
available): beta-lactam antibiotics (5.1.1 Penicillins
and 5.1.2 Cephalosporins and other beta-lactams),
non-beta-lactam antibiotics (5.1 Antibacterial
drugs, other than 5.1.1 and 5.1.2), non-steroidal anti-inflammatory
drugs (10.1.1 NSAIDs), cardiovascular
system (CVS) medications (2 CVS), intravenous
contrast media, allopurinol, opioid analgesic (4.7.2 Opioid analgesics), non-opioid analgesics (4.7.1.
Non-opioid analgesics and compound prep),
antihistamines (3.4.1 Antihistamines), antifungals
(5.2 Antifungal drugs), or others. Patients were
categorised into either geriatric (aged ≥65 years) or
non-geriatric (aged <65 years) groups. Demographic
characteristics, clinical outcomes, and prevalences
of DA labels were compared between groups.
The Chi squared test and independent samples
t tests were respectively used to compare categorical
and continuous variables between groups in
univariate analysis. A P value of <0.05 was considered
statistically significant for the multivariate analysis.
IBM SPSS Statistics for Windows (version 20.0; IBM
Corp, Armonk [NY], Untied States) was used for all
analyses. The study protocol was approved by the
Institutional Review Board of the University of Hong
Kong/Hospital Authority Hong Kong West Cluster.
Results
There were 4361 admissions involving 3641 patients during the 6-month study period. The male-to-female
ratio was 1:1.2. In total, 2522 patients (69.3%)
were included in the geriatric group, with mean age
71.56±17.3 years.
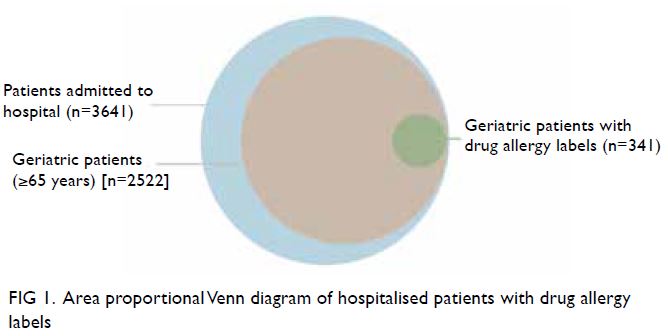
In total, 492 patients (13.5%) had DA labels,
consisting of 151 non-geriatric patients (30.7%) and
341 geriatric patients (69.3%) [Fig 1].
The overall prevalence of DA labels did not significantly differ between geriatric and non-geriatric
patients (13.5% [341/2522] vs 13.5%
[151/1119], P=0.976) [Table]. The absolute and
proportional prevalences of the top 10 categories
of DA labels (in descending order) for geriatric and
non-geriatric patients are shown in Figures 2 and 3, respectively.
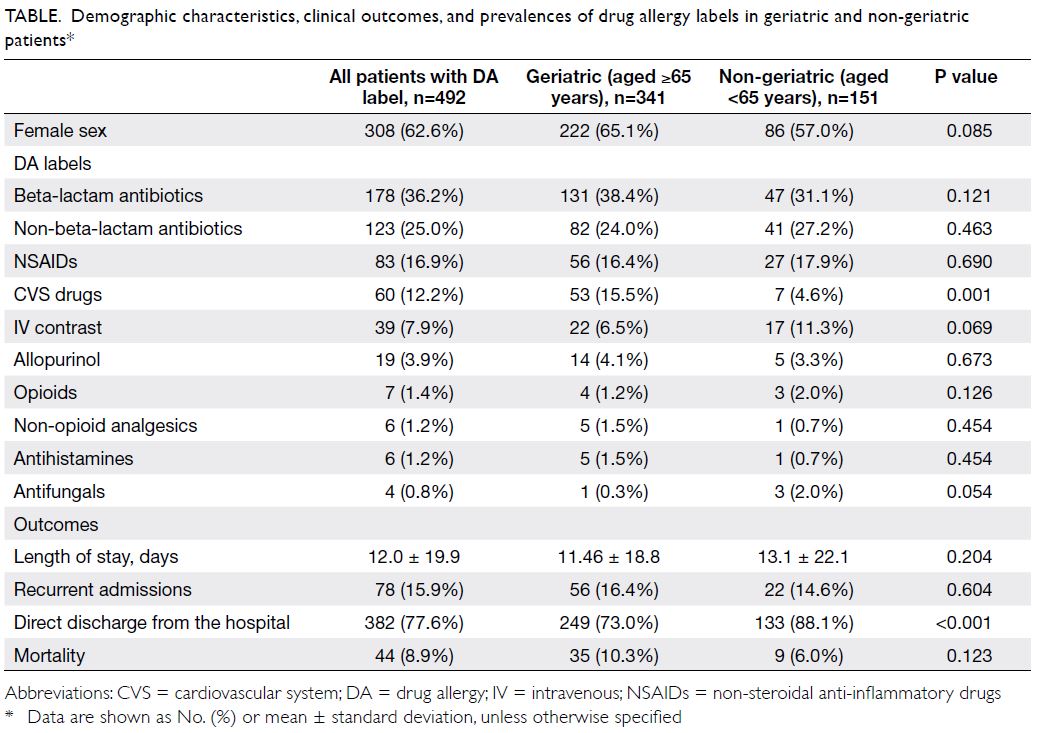
Table. Demographic characteristics, clinical outcomes, and prevalences of drug allergy labels in geriatric and non-geriatric patients
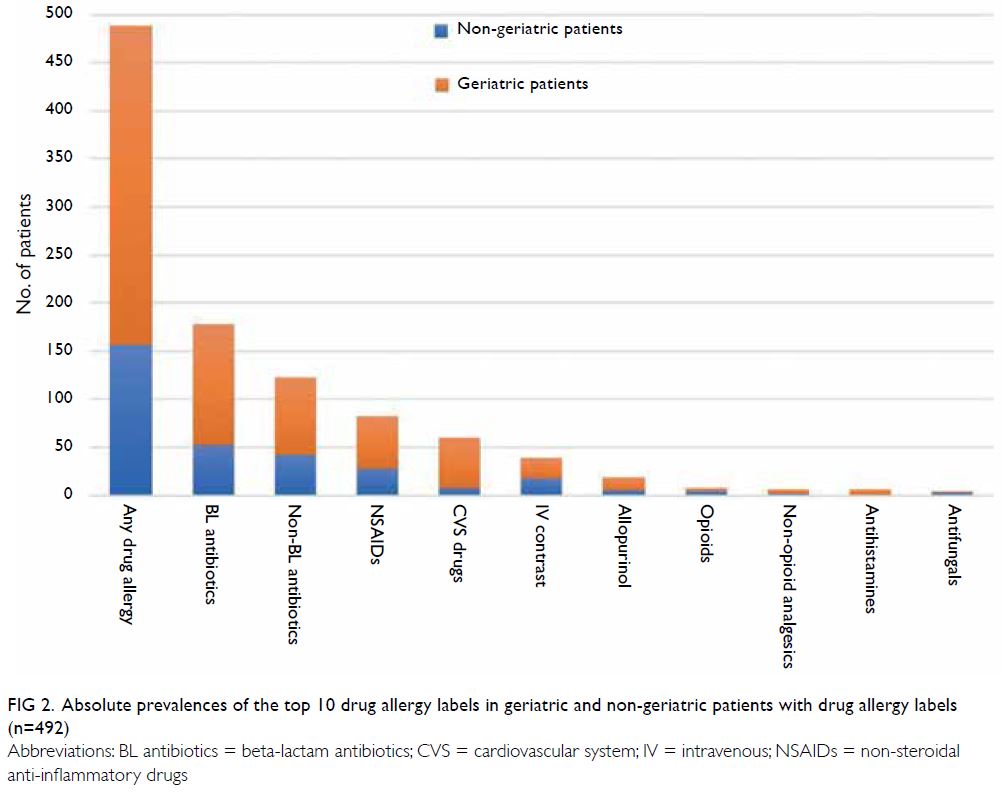
Figure 2. Absolute prevalences of the top 10 drug allergy labels in geriatric and non-geriatric patients with drug allergy labels (n=492)
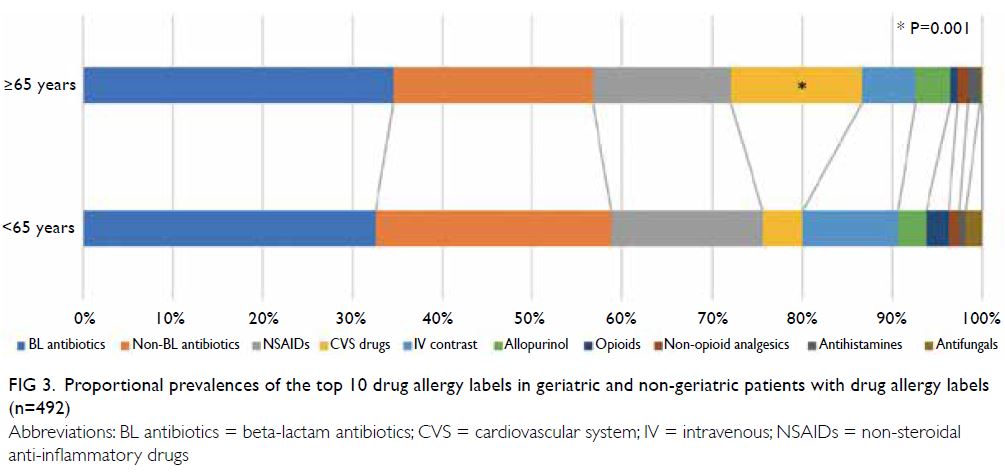
Figure 3. Proportional prevalences of the top 10 drug allergy labels in geriatric and non-geriatric patients with drug allergy labels (n=492)
The majority of patients with DA labels were
aged ≥65 years (69.3%, 341/492). The male-to-female
ratio did not significantly differ between
geriatric and non-geriatric groups. Significantly
more patients in the geriatric group had DA labels
to CVS drugs (15.5% vs 4.6%, P=0.001), while the proportions of other DA labels were similar between
the two groups. Patients in the geriatric group had
a significantly lower rate of direct discharge from
the hospital (73.0% vs 88.1%, P<0.001). The absolute
mortality rate tended to be higher among patients
in the geriatric group, but this difference was not
statistically significant (10.3% vs 6.0%, P=0.123).
Discussion
Although the prevalence and consequences of overall
ADRs have been extensively investigated, this is the
first study to specifically examine the epidemiology
and outcomes of geriatric patients with DA labels. In
our cohort, 13.5% of all hospitalised geriatric patients
had DA labels; the leading causes of DA labels were
comparable between geriatric and non-geriatric
patients. Notably, there were significantly more
labelled DAs to CVS medications and significantly
more adverse clinical outcomes in geriatric patients.
Adverse drug reactions are defined as any
“appreciably harmful or unpleasant reaction”
to medications which can occur through
various immunological or non-immunological
mechanisms.14 Drug allergies or “hypersensitivity
reactions” comprise type B (non-dose-related)
ADRs, which result from specific immune-mediated
responses to a medication. An important problem is
that many non-immune-mediated ADRs are often clinically misinterpreted or incorrectly recorded as
“allergies”. Although the initial DA reactions may
be immunological, genuine allergies may gradually
wane and warrant re-evaluation. For example, the vast majority of patients with beta-lactam allergies
lose skin testing sensitivity over an interval of
10 years.15 16 Similarly, mild delayed (presumptively
T-cell-mediated) reactions do not consistently recur upon re-exposure.17 18 Often, DA labels present in
the medical records of geriatric patients have not
undergone appropriate allergy testing to verify
whether these labels remain accurate. Geriatric
patients also have had more time and events to
become sensitised or develop ADRs which may
be interpreted as allergies; these labels may not
be entirely correct for some patients. Overall, our
study confirms the presence of the high burden of
DA labels in geriatric patients and corresponding
worse clinical outcomes (ie, significantly lower rate
of direct discharge from the hospital) compared with
non-geriatric patients. This highlights the urgent
need to expand the availability of allergy testing for
this vulnerable population.19 20
As expected, beta-lactam antibiotics
constituted the leading cause of DA labels in both
patient populations in our study (61.5% [131/213]
in the geriatric group and 53.4% [47/88] in the non-geriatric
group). In Hong Kong, the prevalence
of reported beta-lactam antibiotic allergy is
approximately 2% with a cumulative incidence
approaching 10 per 100 000 population.21 Beta-lactam
DA labels are known to have clinically
significant consequences including the use of broad-spectrum
antibiotics, enhanced microbial resistance,
greater number of Clostridium difficile infections,
and expansions of multidrug-resistant organisms.10 11 12
In Hong Kong, Chen et al22 found that the prevalence
of methicillin-resistant Staphylococcus aureus was
30.1% among older adults living in residential care
homes. The presence of DA labels greatly restricts the
repertoire of first-line antibiotics for such patients.
Beta-lactams remain the most effective first-line
treatment for many bacterial infections including
methicillin-sensitive Staphylococcus aureus; in
agreement with our findings, the unnecessary use
of alternatives leads to worse patient outcomes,
especially in the vulnerable geriatric population.
Furthermore, we observed a significantly
greater proportion of reported DAs to CVS drugs
among geriatric patients. We postulate that this is
related to the substantially greater burden of CVS
diseases and exposure to CVS drugs in geriatric
patients, compared with other conditions.23 24 As
previously mentioned, although greater exposure
to CVS drugs theoretically increases the risk of
genuine DAs, “allergy” labels could be the result
of incorrectly interpreted ADRs. For example,
the incidences of angiotensin-converting enzyme
inhibitor treatment–related cough and angioedema
(non-immune-mediated ADRs) increase with age.25
Regardless of their accuracy, this greater proportion
of DA labels to CVS drugs is likely to further restrict
therapeutic options and elicit CVS complications in
geriatric patients. The accuracies of these labels and
their specific effects on CVS complications warrant
dedicated studies in the future.
This study had some important limitations.
First, a higher rate of other adverse clinical outcomes
(such as recurrent admissions and mortality) was
evident among patients in the geriatric group,
although this was not statistically significant. This
trend may have constituted a type II statistical
analysis error due to inadequate sampling and
observational design. Second, we only analysed
geriatric and non-geriatric patients with DA labels,
although geriatric patients may have worse clinical
outcomes regardless of DA status. We were also
unable to analyse individual DAs or manifestations
within the CVS subgroup. Nonetheless, our findings
highlight the vulnerability of this specific geriatric
population and emphasise the need for future
prospective studies. Third, although all DAs were
recorded only after confirmation by the patients’
attending doctors and reported manifestations were
screened by an allergist during data collection, we
were unable to ascertain the accuracy of the DA labels.
Comprehensive evaluations of suspected DAs often
require allergological confirmation with skin and/or
drug provocation tests, which is especially difficult
in frail older adults. A follow-up study to identify
the impacts of genuine allergies and incorrectly
interpreted adverse clinical outcomes is currently in
progress. Lastly, the results of our study were from
a single-centre cohort of hospitalised patients and
allergy records may have been influenced by local
physician practices. Additional multicentre studies,
including patients in the ambulatory setting, are
needed to corroborate the external validity of our
findings.
To the best of our knowledge, this is the first
report concerning the epidemiology and outcomes
of geriatric patients with DA labels. More than 13%
of hospitalised geriatric patients had DA labels; the
leading causes of reported DAs in these patients
were similar to those of non-geriatric patients in the
same hospital. We also observed significantly more
reported DAs to CVS drugs, as well as worse clinical
outcomes (ie, more frequent transfer to convalescent
or rehabilitation facilities) among patients in the
geriatric group. Additional dedicated studies are
required to confirm the burden and accuracy of
DA labels among the already-vulnerable geriatric
population.
Author contributions
Concept or design: PH Li.
Acquisition of data: PH Li.
Analysis or interpretation of data: PH Li, HY Chung.
Drafting of the manuscript: PH Li, CS Lau.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: PH Li.
Analysis or interpretation of data: PH Li, HY Chung.
Drafting of the manuscript: PH Li, CS Lau.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Ethical approval was obtained from HKU/HKW Institutional
Review Board of The University of Hong Kong/Hospital
Authority Hong Kong West Cluster (HKU/HA HKW IRB),
Ref UW 18-669.
References
1. Balachandran A, de Beer J, James KS, van Wissen L,
Janssen F. Comparison of population aging in Europe
and Asia using a time-consistent and comparative aging
measure. J Aging Health 2020;32:340-51. Crossref
2. Census and Statistics Department, Hong Kong SAR
Government. Hong Kong Population Projections 2015
Available from: https://www.censtatd.gov.hk/hkstat/sub/sp190.jsp?productCode=B1120015. Accessed 26 May 2020.
3. Pawelec G. Age and immunity: what is “immunosenescence”? Exp Gerontol 2018;105:4-9. Crossref
4. De Martinis M, Sirufo MM, Ginaldi L. Allergy and aging: an old/new emerging health issue. Aging Dis 2017;8:162-75. Crossref
5. Davies EA, O’Mahony MS. Adverse drug reactions in special populations—the elderly. Br J Clin Pharmacol
2015;80:796-807. Crossref
6. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of
prospective studies. JAMA 1998;279:1200-5. Crossref
7. Muehlberger N, Schneeweiss S, Hasford J. Adverse drug
reaction monitoring—cost and benefit considerations.
Part I: frequency of adverse drug reactions causing hospital
admissions. Pharmacoepidemiol Drug Saf 1997;6 Suppl
3:S71-7. Crossref
8. Li PH, Siew LQ, Thomas I, et al. Beta-lactam allergy in
Chinese patients and factors predicting genuine allergy.
World Allergy Organ J 2019;12:100048. Crossref
9. Mattingly TJ 2nd, Fulton A, Lumish RA, et al. The cost of self-reported penicillin allergy: a systematic review. J
Allergy Clin Immunol Pract 2018;6:1649-54.e4. Crossref
10. MacFadden DR, LaDelfa A, Leen J, et al. Impact of reported beta-lactam allergy on inpatient outcomes: a multicenter
prospective cohort study. Clin Infect Dis 2016;63:904-10. Crossref
11. Macy E, Contreras R. Health care use and serious
infection prevalence associated with penicillin “allergy”
in hospitalized patients: a cohort study. J Allergy Clin Immunol 2014;133:790-6. Crossref
12. Blumenthal KG, Lu N, Zhang Y, Li Y, Walensky RP, Choi HK.
Risk of meticillin resistant Staphylococcus aureus and
Clostridium difficile in patients with a documented
penicillin allergy: population based matched cohort study.
BMJ 2018;361:k2400. Crossref
13. Ventura MT, Scichilone N, Paganelli R, et al. Allergic
diseases in the elderly: biological characteristics and main
immunological and non-immunological mechanisms. Clin
Mol Allergy 2017;15:2. Crossref
14. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet
2000;356:1255-9. Crossref
15. Trubiano JA, Adkinson NF, Phillips EJ. Penicillin allergy is not necessarily forever. JAMA 2017;318:82-3. Crossref
16. Blanca M, Romano A, Torres MJ, et al. Update on the evaluation of hypersensitivity reactions to betalactams.
Allergy 2009;64:183-93. Crossref
17. Mori F, Cianferoni A, Barni S, Pucci N, Rossi ME,
Novembre E. Amoxicillin allergy in children: five-day
drug provocation test in the diagnosis of nonimmediate
reactions. J Allergy Clin Immunol Pract 2015;3:375-80.e1. Crossref
18. Bourke J, Pavlos R, James I, Phillips E. Improving the effectiveness of penicillin allergy de-labeling. J Allergy Clin
Immunol Pract 2015;3:365-74.e1. Crossref
19. Chan YT, Ho HK, Lai CK, et al. Allergy in Hong Kong: an unmet need in service provision and training. Hong Kong
Med J 2015;21:52-60. Crossref
20. Lee TH, Leung TF, Wong G, et al. The unmet provision of allergy services in Hong Kong impairs capability for allergy
prevention-implications for the Asia Pacific region. Asian
Pac J Allergy Immunol 2019;37:1-8.
21. Li PH, Yeung HH, Lau CS, Au EY. Prevalence, incidence,
and sensitization profile of beta-lactam antibiotic allergy in
Hong Kong. JAMA Netw Open 2020;3:e204199. Crossref
22. Chen H, Au KM, Hsu KE, et al. Multidrug-resistant
organism carriage among residents from residential
care homes for the elderly in Hong Kong: a prevalence
survey with stratified cluster sampling. Hong Kong Med J
2018;24:350-60. Crossref
23. Islam MM, Valderas JM, Yen L, Dawda P, Jowsey T, McRae IS. Multimorbidity and comorbidity of chronic
diseases among the senior Australians: prevalence and
patterns. PLoS One 2014;9:e83783. Crossref
24. Morgan TK, Williamson M, Pirotta M, Stewart K, Myers SP, Barnes J. A national census of medicines use: a 24-hour
snapshot of Australians aged 50 years and older. Med J
Aust 2012;196:50-3. Crossref
25. Alharbi FF, Kholod AA, Souverein PC, et al. The impact
of age and sex on the reporting of cough and angioedema
with renin-angiotensin system inhibitors: a case/noncase
study in VigiBase. Fundam Clin Pharmacol 2017;31:676-84.Crossref