Hong Kong Med J 2021 Jun;27(3):184–91 | Epub 11 Jun 2021
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Effects of pill splitting training on drug physiochemical properties, compliance, and clinical outcomes in the elderly population: a randomised trial
Vivian WY Lee, PharmD, BCPS1; Joyce TS Li, BPharm1; Felix YH Fong, BPharm1; Bryan PY Yan, FHKAM (Medicine)2
1 Centre for Learning Enhancement and Research, The Chinese University of Hong Kong, Hong Kong
2 Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong
Corresponding author: Prof Vivian WY Lee (vivianlee@cuhk.edu.hk)
Abstract
Introduction: This study aimed to provide information about the clinical and physiochemical
effects of pill splitting training in elderly cardiac
patients in Hong Kong.
Methods: A parallel study design was adopted.
Patients taking lisinopril, amlodipine, simvastatin,
metformin, or perindopril who needed to split pills
were recruited from the Prince of Wales Hospital.
Patients were divided into two groups at their first
visit. Patients in group A split drugs using their
own technique, whereas patients in group B used
pill cutters after relevant training until their next
follow-up visit. The primary outcome was the
change in drug content between before and after
the pill splitting training. Assays were performed to
determine the drug content. Secondary outcomes
were the changes in clinical outcomes, patients’
attitudes and acceptance towards pill splitting, and
patients’ knowledge about pill splitting.
Results: A total of 193 patients were recruited, and
101 returned for the follow-up visit. The percentage
of split tablets falling within the assay limits
increased from 39.13% to 47.82% (P=0.523) in group
A and from 48.94% to 51.06% (P=1.000) in group B. The changes did not reach statistical significance.
As for clinical outcomes, the mean triglyceride level
decreased from 1.62±1.05 to 1.36±0.80 (P=0.049),
whereas the mean heart rate increased significantly
from 73.97±11.01 to 77.92±12.72 (P=0.026). Changes
in other parameters were not significant.
Conclusion: This study highlights the high variability
of drug content after pill splitting. Pills with dosages
that do not require splitting would be preferable,
considering patients’ preference. Patients should be
educated to use pill cutters properly if pill splitting is
unavoidable.
New knowledge added by this study
- There is high variability of drug content after pill splitting.
- Patients prefer to take pills that do not require splitting.
- Patients should be supplied with formulations that do not require splitting if possible.
- Patients should be educated to use pill cutters properly if pill splitting is unavoidable.
Introduction
Pill splitting by patients is common globally. A
German study observed that 24.1% of all drugs
required splitting,1 and a Swiss study found that
10% of all discharged prescriptions contained pill
splitting.2 In Sweden, 10% of 600 000 investigated
prescriptions required splitting, and over 30% of
the Swedish patients stated that they had problems
dividing the tablets.3 The observed prevalence
of pill splitting has been observed to range from
10% to >35% worldwide1 4 5 6 and was even higher in
elderly patients (35-67%).6 7 One of the reasons for pill splitting is cost saving because it may result
in institutions not needing to stock too many
drug items in their formularies.1 In addition, drug
splitting may achieve dose flexibility, particularly
for patients requiring frequent dosing adjustment.8
Furthermore, some dosages may not be commercially
available, especially those for off-label drug use.
In these cases, splitting drugs may be essential.9 10 11
Nevertheless, it can also create other clinical issues
including medication non-compliance, difficulties
experienced by patients in handling unscored pills,
drugs that crumble after splitting, and inappropriate drug splitting of extended release formulations,
which may lead to treatment failure or toxicity.12 A
published study reported that most hypertensive
patients preferred not to split pills and that over 70%
of patients were willing to pay more for medications
with dosages that they did not need to split.13
Limited published studies have addressed this drug-related
problem. In this study, we aimed to identify
the effects of pill splitting on drug physiochemical
properties and clinical outcomes among elderly
cardiac patients.
Methods
Study design
A parallel design was adopted in this study. Patients were recruited from the Cardiac or Hypertension
clinics of the Prince of Wales Hospital, Hong Kong.
After medical records review, it was found that
lisinopril, amlodipine, simvastatin, metformin,
and perindopril were among the most commonly
prescribed medications that required splitting in
the two clinics. Therefore, patients who needed to
split their pills when taking lisinopril, amlodipine,
simvastatin, metformin, or perindopril were
recruited. Patients were randomised into either
group A (pill splitting with self-technique) or group
B (pill splitting with instructions and training) after
their first clinic visit. All patients were asked to sign
an informed consent form before enrolment. Group
A patients were asked to split drugs using their own technique and continue until their next clinic visit.
Group B patients were given proper instruction by
pharmacists or pharmacy students on using pill
cutters at their first visit and were asked to cut their
pills accordingly until their next clinic visit. Patients
watched a 2-minute video that explained the reasons
for using pill cutters and described the proper way
to open the pill cutter, position the drug in the pill
cutter, clean the pill cutter, and store the split pills.
Subsequently, the pharmacist answered patients’
enquiries regarding the video or other questions
related to pill splitting. The current study did not
change any drug or dosage of the patient’s existing
treatment regimens. Follow-up clinic visits were
scheduled with mean duration between first and
follow-up clinic visits of 23.1±7.3 weeks.
Participants
Chinese patients aged ≥65 years, both male and
female, and currently prescribed one or more of
metformin, lisinopril, perindopril, amlodipine, or
simvastatin (which require splitting) were included
in the current study. Patients with dementia or severe
physical limitations such as hemiplegia, blindness,
or upper limb contractures were excluded from the
study.
In our pilot study, we found that the change in
drug assays of metformin, atenolol, and amlodipine
varied from 52.7% to 147.2% after splitting.14 In our
previously published study, systolic blood pressure
decreased significantly (from 152.38±18.80 mm Hg
to 147.04±20.72 mm Hg, P=0.021) after pharmacist
intervention.15 The sample size for the primary
outcome was calculated based on a population
standard deviation of 0.75, and that for the secondary
outcomes was based on a population standard
deviation of 0.85. To achieve a 5% significance level
and 80% power, 30 and 45 patients in each group
would be needed for the primary and secondary
outcomes, respectively. We expected a 10% dropout
rate, and therefore, at least 80 patients were recruited
for each group. There were 193 total participants in
this study.
The participants were randomised into group
A or B using a computerised dynamic allocation
programme and stratification according to types of
medications taken, sex, age, and visit dates to ensure
balanced patient allocation. All operations were
performed by the same pharmacist who conducted
the survey.
Outcome measures
The primary outcome was the change of drug
content before and after the pill splitting training.
At baseline, patients in groups A and B were asked
to split three tablets of the drugs that they were
currently taking using their usual technique. At
follow-up visit, group A patients were asked to split three tablets using their own technique, and group B
patients were asked to split three tablets using a pill
cutter. Two halved tablets were randomly selected for
analysis each time. The halved tablets were weighed,
and standard drug assays were performed. The drug
content of metformin was assessed by ultraviolet-visible
spectrophotometry; that of amlodipine,
simvastatin, and perindopril was assessed by ultra-performance
liquid chromatography; and that of
lisinopril was assessed by high-performance liquid
chromatography.
The secondary outcomes were the changes in
clinical outcomes between before and after the pill
splitting training, including the change in blood
pressure measurements, haemoglobin A1c, and
cholesterol levels, the changes in patients’ attitudes
towards and acceptance of pill splitting, and the
changes in patients’ knowledge about pill splitting.
Haemoglobin A1c and lipid levels were usually
collected in hospital 1 week before patients’ clinic
visit. Upon initiating their clinic visits, patients had
their blood pressures measured in the hospital’s
nurse station before they met their physicians. Blood
pressure, haemoglobin A1c, and cholesterol levels
were collected at baseline and at follow-up visit, and
the patients’ knowledge about and attitudes towards
pill splitting were assessed by questionnaires.
Statistical analysis
Paired-samples t tests were used for intra-group comparisons, and independent-samples t tests
were used for inter-group comparisons of mean
tablet weight between before and after pill splitting
training. Paired-samples t tests were also used to
assess the changes in clinical outcomes. McNemar’s
test was used for intra-group comparisons, and
Fisher’s exact test (two-sided) was used for inter-group
comparisons of content uniformity, change
in patients’ acceptance towards pill splitting, and
change in patients’ knowledge about pill splitting.
A P value of <0.05 was considered statistically
significant. All analyses were performed using the
SPSS statistical programme (Windows version 25.0;
IBM Corp, Armonk [NY], United States).
Results
Participants
A total of 193 eligible patients were enrolled on or before 17 January 2019, and they had follow-up
visits on or before 30 April 2019. The patients were
randomised into group A (n=106) and group B (n=87).
A total of 101 patients participated, of whom 47 from
group A and 54 from group B returned for follow-up
visit. Among them, 46 patients from group A and 47
patients from group B provided samples for assay.
The primary outcome analysis was conducted on
those patients, and the secondary outcomes analysis was conducted on the 101 patients who returned for
follow-up visit. The patients’ demographic data are
shown in Table 1.
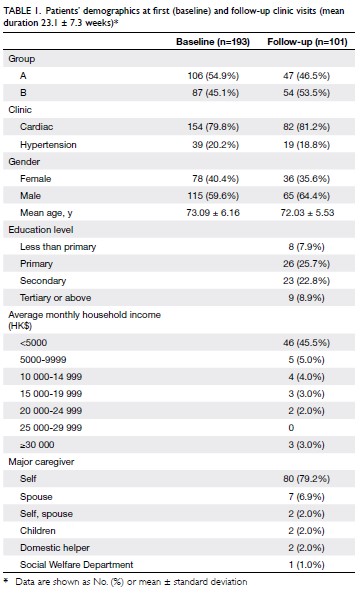
Table 1. Patients’ demographics at first (baseline) and follow-up clinic visits (mean duration 23.1 ± 7.3 weeks)
Drug content
The primary outcome was the change of drug content between before and after the pill splitting training.
Patients were asked to split three tablets during each
visit. Two halved tablets were randomly selected as
samples. The samples were weighed, and assays were
performed. The mean weight of the halved tablets
at baseline and at follow-up visit is documented
in Table 2. Table 3 shows the percentage of halved
tablets that were within the assay specifications at
baseline and at follow-up visit. The percentage of samples with both halved tablets within range was
compared between groups A and B. In group A,
the percentage in range increased from 39.13% to
47.82% (P=0.523), and the corresponding increase
for group B was from 48.94% to 51.06% (P=1.000).
The difference in drug assay results between groups
A and B at baseline (P=0.406) and at follow-up visit
(P=0.837) also did not reach statistical significance.
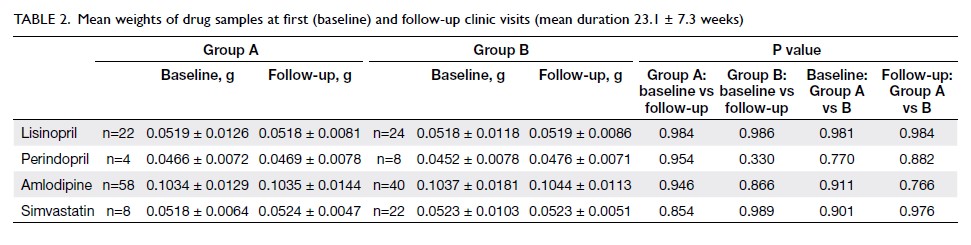
Table 2. Mean weights of drug samples at first (baseline) and follow-up clinic visits (mean duration 23.1 ± 7.3 weeks)
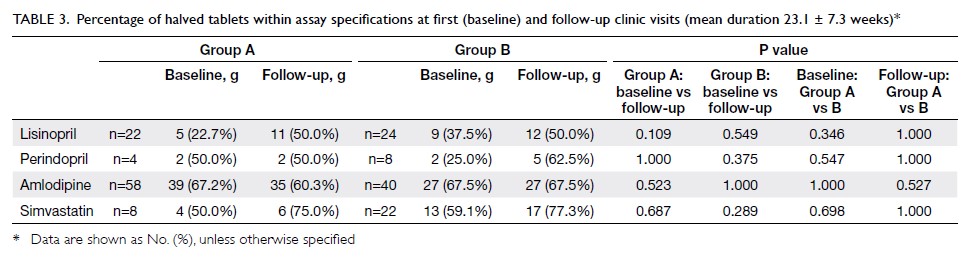
Table 3. Percentage of halved tablets within assay specifications at first (baseline) and follow-up clinic visits (mean duration 23.1 ± 7.3 weeks)
Clinical outcomes
The correlation between pill cutting training and
clinical outcomes is summarised in Table 4. The
mean triglyceride in group B decreased significantly from 1.62±1.05 to 1.36±0.80 mmol/L (P=0.049),
whereas the mean heart rate increased significantly
from 73.97±11.01 to 77.92±12.72 bpm (P=0.026).
In group B, there was also improvement in the
mean diastolic blood pressure (from 73.40±14.39
to 73.05±9.33 mm Hg), high-density lipoprotein
(from 1.40±0.39 to 1.46±0.44 mmol/L), low-density
lipoprotein (from 1.87±0.88 to 1.85±0.73 mmol/L),
and total cholesterol (from 3.98±0.93 to
3.91±0.85 mmol/L), but those differences did
not reach statistical significance. In the overall
cohort, improvements were seen in diastolic blood
pressure (from 74.21±12.47 to 74.01±9.98 mm Hg), high-density lipoprotein (from 1.42±0.37 to
1.43±0.40 mmol/L), total cholesterol (from
3.90±0.97 to 3.88±0.96 mmol/L), and triglyceride
(from 1.46±0.91 to 1.31±0.72 mmol/L), but the
changes did not reach statistical significance.
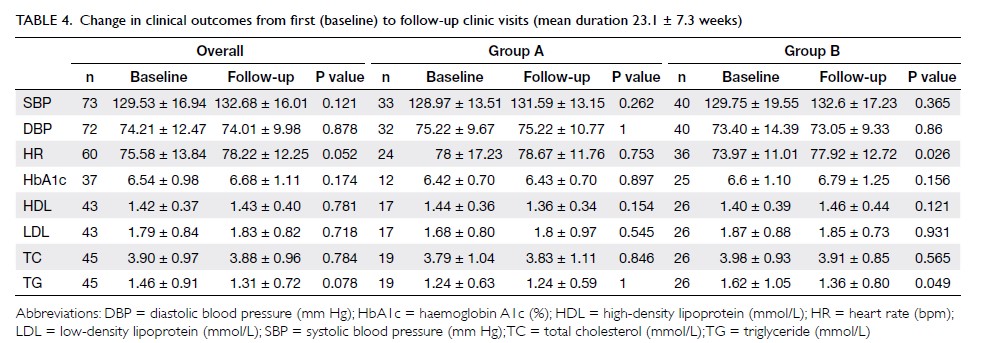
Table 4. Change in clinical outcomes from first (baseline) to follow-up clinic visits (mean duration 23.1 ± 7.3 weeks)
Patients’ backgrounds, attitudes, and
knowledge
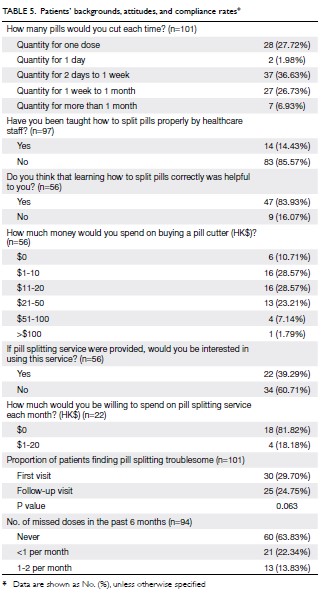
In total, 57.43% of patients split their pills with their bare hands, followed by pill cutters (24.75%), knives
(13.86%), and scissors (10.89%). The major reasons
for not using pill cutters included: (1) the current
method could split pills evenly (68.18%), (2) using
pill cutters was time consuming (34.09%), and (3) the
pills could not be split evenly by pill cutters (15.91%).
The major reasons for using pill cutters included:
(1) pills could be cut evenly (80.95%) and (2) the
patient was able to exert force more easily (33.33%).
In total, 29.70% and 24.75% of patients found pill
splitting troublesome at baseline and at follow-up
visit, respectively (the difference was not significant,
P=0.063). The three major problems encountered
by patients while splitting pills were (1) difficulty
splitting the pills evenly (17.00%), (2) the pills easily
fragmented (10.00%), and (3) difficulty seeing the
pills clearly, as they were too small (9.00%). Overall,
61.00% of the patients claimed that they had no
difficulties. Nevertheless, 98.21% preferred to take
tablets with exact dosages so that no splitting would
be required. Patients’ responses to other questions
are listed in Table 5.
Table 6 shows that a significantly higher portion
of patients in group B had a correct understanding of
the following three questions after training: ‘Using
pill cutters allows pills to be divided into more
accurate doses’ (from 7.41% to 31.48%; P=0.002);
‘The pills should be put into the triangular tip of
the pill cutter’ (from 9.26% to 31.48%; P=0.008); and
‘Pill cutters should be stored in a cool and dry place,
away from sun or moisture’ (from 9.26% to 35.19%;
P=0.003). In contrast, patients in group A did not
show a statistically significant improvement in their
understanding of any question. During the interview
and evaluation of patients’ knowledge about pill
splitting at baseline and at follow-up visit, we did not
detect any patients with major physical or cognitive
abnormalities.
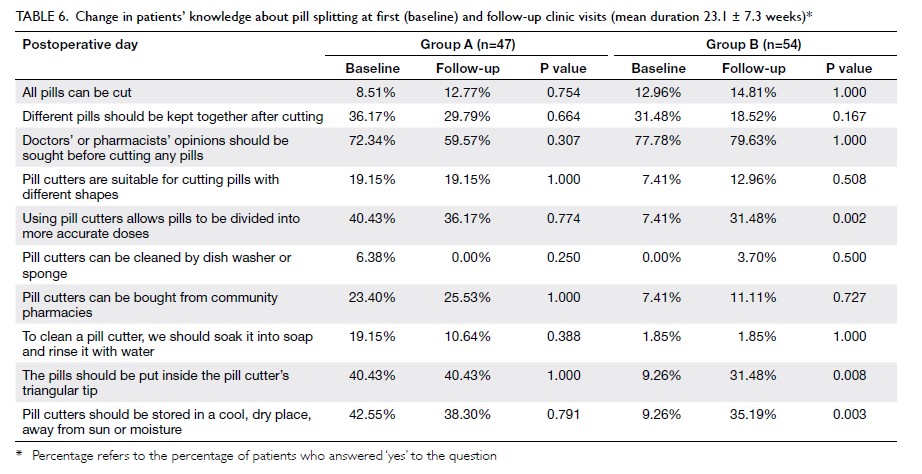
Table 6. Change in patients’ knowledge about pill splitting at first (baseline) and follow-up clinic visits (mean duration 23.1 ± 7.3 weeks)
Discussion
Tablet splitting is a common practice in in-patient and out-patient settings,16 and it may be desirable
in terms of dose adjustment, cost saving, and
ease of swallowing.3 11 12 17 18 19 20 Nevertheless, it has
been reported that splitting pills may cause drug
instability, loss of drug due to powdering, uneven
dosage, and reduced drug strength.21 22 It is generally
understood that using tablet splitting devices can
provide a more consistent dose.10 21 Previous studies have identified some characteristics that might affect
the quality of halved tablets. Coated, unscored, and
small tablets were found to be more difficult to cut.23
Individual pill cutting skill was another crucial factor
that determined tablets’ uniformity.23 In the current
study, only 24.75% of patients split pills using pill
cutters, and only 14.43% of patients had received
pill splitting training. Therefore, it is likely that the
drug content in the halved tablets did not reach assay
standards.
Previous studies mainly focused on the weight
deviations among halved tablets, not on drug
content.21 22 One study showed that more than
one-third of sampled half-tablets did not meet the United
States Pharmacopeia specifications.24 The measured
drug content variations among half-tablets were:
warfarin sodium (90.01%-109.40%), simvastatin
(95.21%-111.35%), metoprolol succinate (82.77%-115.92%), metoprolol tartrate (94.83%-112.37%),
citalopram (96.50-111.93%), and lisinopril (81.15%-125.72%). In another study, five of eight drugs failed
to meet European Pharmacopoeia recommendations
for tablet weight deviation after splitting, with 25%
of samples deviating by >15% and 10% of samples
deviating by >25%.23 The study drugs used were
phenobarbitone (maximum deviation: 80.45%),
digoxin (maximum deviation: 56.69%), chloroquine
(maximum deviation: 48.97%), atenolol (maximum
deviation: 45.37%), and doxycycline (maximum
deviation: 43.97%). In the present study, both halves
of the tablet were within the assay standard at baseline
for 39.13% and 48.94% of the patients in groups A
and B, respectively. After training, this percentage
increased to 47.82% and 51.06%, respectively, but the
improvement was not significant, and the percentage
of tablets in range was still relatively low. The results
corroborated those of previous studies.
Few studies have examined the effect of patient
education on the drug content of split pills.25 26 In the
current study, we found no significant improvement
in content uniformity after pill splitting training. This
may be because our patients were elderly patients who may not have been able to perform the task well
after a single training session. Content uniformity
after pill splitting may be improved if pills are split by
pharmacists or qualified staff. A study of paediatric
pharmacists suggested that tablets >8 mm could be
split once to achieve an approximate half dose for
paediatric use.27 Another study found a significant
difference in splitting accuracy between nurses
and laypersons.23 Nevertheless, only 39.29% of that
study’s patients were eager to partake of pill splitting
service, and only 18.18% were willing to pay extra
money for it. Therefore, pill cutting service may not
be practical without a financial incentive.
Triglyceride levels decreased significantly
and heart rate increased significantly in group B
patients after the intervention. Nevertheless, we
did not evaluate the patients’ diet consumption or
exercise levels, which may impact their triglyceride
levels. No significant change in clinical outcomes
was observed in other groups or other parameters.
Because the studied drugs were lisinopril,
perindopril, simvastatin, and amlodipine, which
are not narrow-therapeutic-index drugs, these
results were predictable and coincide with other
studies that concluded that drugs with long half-life
and wide therapeutic index are less likely to be
affected.20 28 In view of the high variability of blood
pressure measurements in the clinic, all patients
were originally instructed to conduct daily blood
pressure measurements at home using a portable
blood pressure monitor. However, many patients did
not measure their blood pressure daily or did not keep a proper self-record, so the clinical outcomes
relied on the readings at clinic visits, which may not
be consistent with their usual readings. In addition,
management of chronic diseases like hypertension,
diabetes mellitus, and dyslipidaemia could be
influenced by multiple factors, and 3 months was
a relatively short period for observation. The effect
of drug content deviation after splitting on clinical
outcomes may be more obvious in antibiotics or drugs
with narrow therapeutic index (eg, digoxin).23 29 30
In the current study, we focused on the effect
of pill splitting on drug content. Nevertheless, pill
splitting may have other effects on drugs. The pill
may carry a bitter taste, as the coating is broken,
and the active ingredients may be more susceptible
to moisture after exposure.31 Over 70% of patients
prepared a sufficient quantity of pills for more than
1 day each time. In total, 36.63% of patients cut for
2 days to 1 week, 26.73% cut for 1 week to 1 month,
and 6.93% cut for more than 1 month each time.
Exposing the cut pills for too long may increase the
risk of crushing or cracking.19 In total, 83.93% of
patients found pill splitting training helpful, and the
intervention produced significant improvement in
patients’ knowledge about pill splitting. This study
has identified the major difficulties encountered by
patients and the reasons behind their choices. Those
problems should be addressed in future patient
education. More than half of patients split pills with
their bare hands, and the majority of patients who did
not use pill cutters thought their own methods could
divide pills evenly and that the use of pill cutters
was time consuming. The major obstacles patients
faced were the difficulties in splitting pills evenly
and that the pills fragmented easily. Overall, 98.21%
of patients preferred to take tablets with the exact
dosage instead of splitting pills. Previous studies
also found that dispensing the exact dosage would
be more favourable.23 32 Nevertheless, if pill splitting
is unavoidable, pharmacists should encourage
patients to split coated, unscored, or irregularly
shaped tablets with pill cutters to reduce crushing
or fragmenting. Pharmacists should also educate
patients about the appropriate way to use and clean
pill cutters and remind patients to seek doctors’ or
pharmacists’ advice before cutting any pills.21 23 33
This project has several limitations. First, the
participants’ dropout rate was high, which might
result in attrition bias. Compared with group A, a
higher proportion of group B patients returned
for follow-up visits. The statistically significant
improvement in clinical outcomes among group B
patients might be caused by their higher awareness
about their own health instead of the effectiveness of
the pill splitting method. There were limited human
resources to make phone calls to patients between
the baseline and face-to-face follow-up visits, which
could have served as a reminder for patients to attend follow-up visits and perform home monitoring of
their blood pressure and their pill splitting methods.
Second, dietary consumption and exercise
levels were not evaluated, even though they may affect
the clinical outcomes. Third, participants’ education
level, household income, and major caregivers were
not collected at baseline. Only approximately 65% of
participants who attended follow-up visits provided
such information. These confounding factors might
affect patients’ ability to understand and memorise
the steps of using pill cutters, thus affecting the
content uniformity of their split pills. The effects of
patients’ characteristics on their knowledge and pill
splitting skills were not assessed in the current study.
Conclusion
This study revealed that content uniformity can
hardly be achieved after pill splitting by patients.
No significant difference in clinical outcomes was
observed after pill splitting training. It is preferable
for pills with doses that do not require splitting
to be provided, considering the assay results and
patients’ preference. Currently, there is inadequate
patient education about pill splitting. Pharmacists
should educate patients to use pill cutters properly if
splitting is inevitable.
Author contributions
Concept or design: VWY Lee, FYH Fong, BPY Yan.
Acquisition of data: VWY Lee, FYH Fong, BPY Yan.
Analysis or interpretation of data: JTS Li.
Drafting of the manuscript: JTS Li.
Critical revision of the manuscript for important intellectual content: VWY Lee.
Acquisition of data: VWY Lee, FYH Fong, BPY Yan.
Analysis or interpretation of data: JTS Li.
Drafting of the manuscript: JTS Li.
Critical revision of the manuscript for important intellectual content: VWY Lee.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
As an editor of the journal, BPY Yan was not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Acknowledgement
We thank the technicians at the School of Pharmacy, The
Chinese University of Hong Kong for carrying out the drug
physiochemical tests and the staff at Prince of Wales Hospital
for arranging the logistics for patient counselling.
Funding/support
This study was supported by the Health and Medical Research Fund, Food and Health Bureau, Hong Kong SAR Government
(#14152111). The funder had no role in study design, data
collection/analysis/interpretation, or manuscript preparation.
Ethics approval
Ethical approval was obtained from The Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee (Clinical trial registration
no.: CREC Ref No. 2017.014). Patient consent was obtained
upon enrolment. The trial protocol can be obtained as
requested.
References
1. Quinzler R, Gasse C, Schneider A, Kaufmann-Kolle P, Szecsenyi J, Haefeli WE. The frequency of inappropriate
tablet splitting in primary care. Eur J Clin Pharmacol
2006;62:1065-73. Crossref
2. Berg C, Ekedahl A. Dosages involving splitting tablets: common but unnecessary? J Pharm Health Serv Res
2010;1:137-41. Crossref
3. Rodenhuis N, De Smet PA, Barends DM. The rationale of scored tablets as dosage form. Eur J Pharm Sci 2004;21:305-
8. Crossref
4. Rodenhuis N, de Smet PA, Barends DM. Patient experiences with the performance of tablet score lines
needed for dosing. Pharm World Sci 2003;25:173-6. Crossref
5. Stanković I, Pešić M, Lalić M. Breaking of tablets as a cost-saving strategy. Timočki Medicinski Glasnik 2007;32:5-10.
6. Fischbach MS, Gold JL, Lee M, Dergal JM, Litner GM, Rochon PA. Pill splitting in a long-term care facility. CMAJ
2001;164:785-6.
7. Denneboom W, Dautzenberg MG, Grol R, De Smet PA.
User-related pharmaceutical care problems and factors
affecting them: the importance of clinical relevance. J Clin
Pharm Ther 2005;30:215-23. Crossref
8. Peek BT, Al-Achi A, Coombs SJ. Accuracy of tablet splitting by elderly patients. JAMA 2002;288:451-2. Crossref
9. Duncan MC, Castle SS, Streetman DS. Effect of tablet splitting on serum cholesterol concentrations. Ann
Pharmacother 2002;36:205-9. Crossref
10. Rindone JP. Evaluation of tablet-splitting in patients taking lisinopril for hypertension. JCOM Wayne PA 2000;7:22-30.
11. Gee M, Hasson NK, Hahn T, Ryono R. Effects of a tablet-splitting
program in patients taking HMG-CoA reductase
inhibitors: analysis of clinical effects, patient satisfaction,
compliance and cost avoidance. J Manag Care Pharm
2002;8:453-8. Crossref
12. Fawell NG, Cookson TL, Scranton SS. Relationship
between tablet splitting and compliance, drug acquisition
costs, and patient acceptance. Am J Health Syst Pharm
1999;56:2542-5. Crossref
13. McDevitt JT, Gurst AH, Chen Y. Accuracy of tablet
splitting. Pharmacotherapy 1998;18:193-7.
14. Lee VW, Yu Y, Tang L, Yan B. Impact of pill-splitting
training on drug physiochemical properties, compliance
and clinical outcomes in elderly population–a cross-over
cohort study. Value Health 2013;16:A514. Crossref
15. Lee VW, Pang PT, Kong KW, Chan PK, Kwok FL.
Impact of pharmacy outreach services on blood pressure
management in the elderly community in Hong Kong.
Geriatr Gerontol Int 2013;13:175-81. Crossref
16. Crawford DJ. Is tablet splitting safe? Nursing made
incredibly easy 2011;9:18-20. Crossref
17. Cohen CI, Cohen SI. Potential savings from splitting newer antidepressant medications. CNS Drugs 2002;16:353-8. Crossref
18. Stafford RS, Radley DC. The potential of pill splitting to achieve cost savings. Am J Manag Care 2002;8:706-12.
19. Bachynsky J, Wiens C, Melnychuk K. The practice of splitting tablets: cost and therapeutic aspects.
Pharmacoeconomics 2002;20:339-46. Crossref
20. Helmy SA. Tablet splitting: is it worthwhile? Analysis of
drug content and weight uniformity for half tablets of 16
commonly used medications in the outpatient setting. J
Manag Care Spec Pharm 2015;21:76-86. Crossref
21. Verrue C, Mehuys E, Boussery K, Remon JP, Petrovic M.
Tablet-splitting: a common yet not so innocent practice. J
Adv Nurs 2010;67:26-32. Crossref
22. Rosenberg JM, Nathan JP, Plakogiannis F. Weight variability
of pharmacist-dispensed split tablets. J Am Pharm Assoc
(Wash) 2002;42:200-5. Crossref
23. Elliott I, Mayxay M, Yeuichaixong S, Lee SJ, Newton PN.
The practice and clinical implications of tablet splitting in
international health. Trop Med Int Health 2014;19:754-60. Crossref
24. Hill SW, Varker AS, Karlage K, Myrdal PB. Analysis of
drug content and weight uniformity for half-tablets of
6 commonly split medications. J Manag Care Pharm
2009;15:253-61. Crossref
25. Gharaibeh SF, Tahaineh L. Effect of different splitting
techniques on the characteristics of divided tablets of five
commonly split drug products in Jordan. Pharm Pract
(Granada) 2020;18:1776. Crossref
26. Ashrafpour R, Ayati N, Sadeghi R, et al. Comparison of
treatment response achieved by tablet splitting versus
whole tablet administration of levothyroxine in patients
with thyroid cancer. Asia Ocean J Nucl Med Biol
2018;6:108-12. Crossref
27. Andersson ÅC, Lindemalm S, Eksborg S. Dividing the
tablets for children—good or bad? Pharm Methods
2016;7:23-7. Crossref
28. Chou CL, Hsu CC, Chou CY, Chen TJ, Chou LF, Chou YC. Tablet splitting of narrow therapeutic index
drugs: a nationwide survey in Taiwan. Int J Clin Pharm
2015;37:1235-41. Crossref
29. Simpson JA, Watkins ER, Price RN, Aarons L, Kyle DE, White NJ. Mefloquine pharmacokinetic-pharmacodynamic
models: implications for dosing and resistance. Antimicrob
Agents Chemother 2000;44:3414-24. Crossref
30. White NJ, Pongtavornpinyo W, Maude RJ, et al. Hyperparasitaemia and low dosing are an important
source of anti-malarial drug resistance. Malar J 2009;8:253. Crossref
31. Margiocco ML, Warren J, Borgarelli M, Kukanich B.
Analysis of weight uniformity, content uniformity and
30-day stability in halves and quarters of routinely
prescribed cardiovascular medications. J Vet Cardiol
2009;11:31-9. Crossref
32. Madathilethu J, Roberts M, Peak M, Blair J, Prescott R,
Ford JL. Content uniformity of quartered hydrocortisone
tablets in comparison with mini-tablets for paediatric
dosing. BMJ Paediatr Open 2018;2:e000198. Crossref
33. Allemann SS, Bornand D, Hug B, Hersberger KE, Arnet I.
Issues around the prescription of half tablets in Northern
Switzerland: the irrational case of quetiapine. Biomed Res
Int 2015;2015:602021. Crossref