Hong Kong Med J 2021 Jun;27(3):177–83 | Epub 19 Feb 2021
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Expanded carrier screening using next-generation
sequencing of 123 Hong Kong Chinese families:
a pilot study
Olivia YM Chan, FHKCOG, FHKAM (Obstetrics and Gynaecology)1,2 #; TY Leung, FRCOG, FHKAM (Obstetrics and Gynaecology)1,3 #; Y Cao, PhD1,3,4; MM Shi, MPhil1; Angel HW Kwan, MRCOG1; Jacqueline PW Chung, FHKCOG, FHKAM (Obstetrics and Gynaecology)1; KW Choy, PhD1,3; SC Chong, FHKCPaed, FHKAM (Paediatrics)3,4
1 Department of Obstetrics and Gynaecology, The Chinese University of Hong Kong, Hong Kong
2 Adept Medical Centre, Hong Kong
3 The Chinese University of Hong Kong–Baylor College of Medicine Joint Center of Medical Genetics, Hong Kong
4 Department of Paediatrics, The Chinese University of Hong Kong, Hong Kong
# These authors equally contributed to this work
Corresponding author: Dr SC Chong (chongsc@cuhk.edu.hk)
Abstract
Introduction: To determine the carrier frequency
and common mutations of Mendelian variants in
Chinese couples using next-generation sequencing
(NGS).
Methods: Preconception expanded carrier testing
using NGS was offered to women who attended
the subfertility clinic. The test was then offered to
the partners of women who had positive screening
results. Carrier frequency was calculated, and the
results of the NGS panel were compared with those of
a target panel.
Results: In total, 123 women and
20 of their partners were screened. Overall, 84 (58.7%)
individuals were identified to be carriers of at least
one disease, and 68 (47.6%) were carriers after
excluding thalassaemias. The most common diseases
found were GJB2-related DFNB1 nonsyndromic
hearing loss and deafness (1 in 4), alpha-thalassaemia
(1 in 7), beta-thalassaemia (1 in 14), 21-hydroxylase
deficient congenital adrenal hyperplasia (1 in 13),
Pendred’s syndrome (1 in 36), Krabbe’s disease (1 in
48), and spinal muscular atrophy (1 in 48). Of the
43 identified variants, 29 (67.4%) were not included
in the American College of Medical Genetics and
Genomics or American College of Obstetrics and
Gynecology guidelines. Excluding three couples
with alpha-thalassaemia, six at-risk couples were
identified.
Conclusion: The carrier frequency of the investigated
members of the Chinese population was 58.7%
overall and 47.6% after excluding thalassaemias.
This frequency is higher than previously reported.
Expanded carrier screening using NGS should be
provided to Chinese people to improve the detection
rate of carrier status and allow optimal pregnancy
planning.
New knowledge added by this study
- The carrier frequency of Mendelian variants in the Chinese population is higher than previously reported.
- Next-generation sequencing should be used in the Chinese population to increase the detection rate of carriers of Mendelian variants.
- Expanded carrier screening with next-generation sequencing should be provided to Chinese people to identify carrier status of Mendelian variants for pregnancy planning.
Introduction
Carrier screening aims to identify couples at risk
of conceiving children affected by recessive genetic
diseases. Carrier couples of most recessive genetic
conditions are typically asymptomatic, and the
only way to identify them is by carrier screening.
If a couple are both carriers of the same autosomal
recessively inherited condition, their offspring have a 1 in 4 chance of being affected. The risk is as high as
1 in 2 in male offspring if the mother is an X-linked
recessive carrier. Carrier screening facilitates
informed prenatal testing options such as pre-implantation
genetic diagnosis, prenatal invasive
testing, and other reproductive options such as
donor gametes and adoption for carrier couples.
Prenatal genetic diagnosis could provide parents with more information, appropriate counselling, and
preparation to take care of the child.1
Various carrier screening programmes
targeting specific populations have been developed
for single gene diseases such as cystic fibrosis,
thalassaemia, and Tay-Sachs disease.2 3 The
American College of Obstetrics and Gynecology
(ACOG) published guidelines on ethnically based
carrier screening programmes, eg, screening for
haemoglobinopathies in individuals of Southeast
Asian, African and Mediterranean descent and
screening for cystic fibrosis, Tay-Sachs disease,
familial dysautonomia, and Canavan disease for
individuals of Ashkenazi Jewish descent.2 4 However,
race and ethnicity can only be determined by patient
self-report, and measures to ascertain ethnicity
are restrictive.5 Ancestry-based screening could
also lead to unequal distribution of genetic testing
and may miss diagnosis of diseases in populations
without screening.3 Thus, both the American College
of Medical Genetics and Genomics (ACMG) and
ACOG recommended carrier screening for cystic
fibrosis in all couples in 2001.6 7 The ACMG and
ACOG have also recommended carrier screening for
spinal muscular atrophy (SMA) in all couples since
2008 and 2017, respectively.8 9
With advancements in genomic technology
providing access to next-generation sequencing
(NGS), expanded screening panels that cover a wide variety of disorders could be offered to individuals
regardless of ethnic background.9
The common mutations in the screening panel
are mainly chosen based on studies performed in the
Caucasian and Ashkenazi Jewish populations. Those
known common mutations may not be ethnicity-specific
and may not cover all mutations present
in the Chinese population. Thus, the approach of
sequencing the entire disease-causing gene would be
more useful than the targeted common mutations
approach for the Chinese population.
Studies that evaluate carrier frequencies and
common mutations in the Chinese population are
lacking in our locality. Further study to review carrier
frequency and the identified variants in the Chinese
population is essential to guide the future design of
carrier screening platforms specific to the Chinese
population and improve the cost-effectiveness of
carrier screening for genetic diseases.
Methods
Subjects
Expanded carrier screening testing was offered to
women who attended the subfertility clinic and
pre-pregnancy counselling clinic of the study unit
between March 2016 and March 2017. They were
counselled about the prevalence and inheritance
of recessive conditions, and the chance of having
affected offspring for a silent carrier couple, using
examples and figures. The purpose, testing methods,
interpretation of results, potential benefits, risks,
and limitations of the expanded carrier screening
were also explained.
A generic consent form for the expanded
carrier screening testing prepared by the laboratory
was used. Consent for the use of data obtained
for research or audit purposes was also obtained.
The test was then ordered by the clinician as self-financed
testing. The expanded carrier screening
test was offered to both members of the couple
separately during pre-test counselling. During post-test
counselling, if a woman was identified to be a
carrier of an autosomal recessive disease, but her
partner had not completed the test, her partner was
also counselled for carrier testing using the same
method as self-financed testing. If both the male and
female members of the couple were carriers of a same
autosomal recessive disorder or the female was the
carrier of an X-linked recessive disorder, they were
identified as at-risk couples having the possibility
of an affected pregnancy. Genetic counselling was
arranged for at-risk couples to discuss reproductive
options such as preimplantation genetic testing
and prenatal diagnostic testing. Finally, the carrier
frequencies of individual diseases and the identified
variants were reviewed. STROBE reporting
guidelines were implemented in this manuscript.
Disease panels
The expanded carrier screening panel consisted of
104 conditions inherited in autosomal recessive or
X-linked manner (online supplementary Appendix).
The severity of these conditions ranged from
debilitating diseases with neurological impairment
(eg, SMA), reduced lifespan (eg, thalassaemia), or
intellectual disability (eg, fragile X syndrome) to
diseases requiring early intervention in the prenatal
or early neonatal period (eg, 21-hydroxylase deficient
congenital adrenal hyperplasia [CAH]).
Laboratory tests
The screening platform (Family Prep Screen 2.0;
Counsyl, South San Francisco [CA], United States),
which was reported by Lazarin et al,10 uses NGS
techniques to analyse the listed exons, as well as
selected intergenic and intronic regions, of the
genes responsible for the recessive conditions. The
selected regions were sequenced to high coverage
and compared with standards and references of normal
variation. High-throughput sequencing detects
approximately 94% of known clinically significant
variants according to the test provider. Variants
classified as ‘predicted’ or ‘likely’ pathogenic have
been reported.11 Fragile X specific polymerase chain
reaction assay was used to determine the CGG
repeat size in the 5' untranslated region of the FMR1
gene. Targeted copy number analysis was used to
determine the copy number of exon 7 of the SMN1
gene. g.27134T>G variant testing for identification of
silent SMA carriers is not included in this platform.12
The turnaround time of the test was approximately
3 weeks.
Results
A total of 123 Chinese women (age range, 20-45 years)
opted for expanded carrier screening, and 69 (56.1%)
of them were found to be carriers of at least one
disease. Twenty of the women’s partners (29.0%,
20/69) were willing to complete the screening test
after genetic counselling. Screening for possible
carrier status before contemplating pregnancy was
the indication in all individuals. Excluding one
woman who was positive for fragile X syndrome,
48 women who screened positive opted not to
screen their partners. Seventeen of them were solely
carriers of alpha- or beta-thalassaemia (10 and 7, respectively), which could be accurately screened
by mean corpuscular volume. The results also
included 20 GJB2 carriers, especially the c.109G>A
(p.Val37Ile) mutation, which has low penetrance and
is prevalent in the Chinese population.13 14 Carrier
status for CAH, SMA, Pendred’s syndrome, and
other very rare diseases was found in three, one, one,
and six individuals, respectively. After integrating
partners’ data, 84 subjects (58.7%) were found to be
carriers for at least one recessive disease, including
thalassaemias. Excluding thalassaemias, 68 subjects
(47.6%) were found to be carriers of at least one
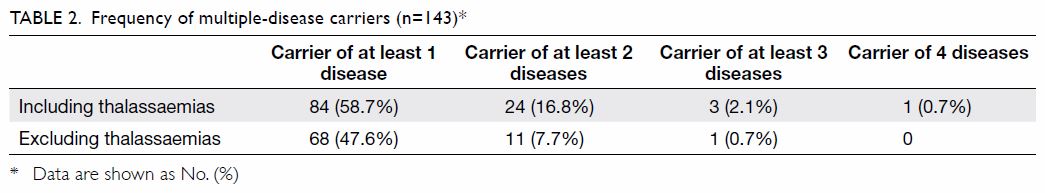
disease (Tables 1 and 2).
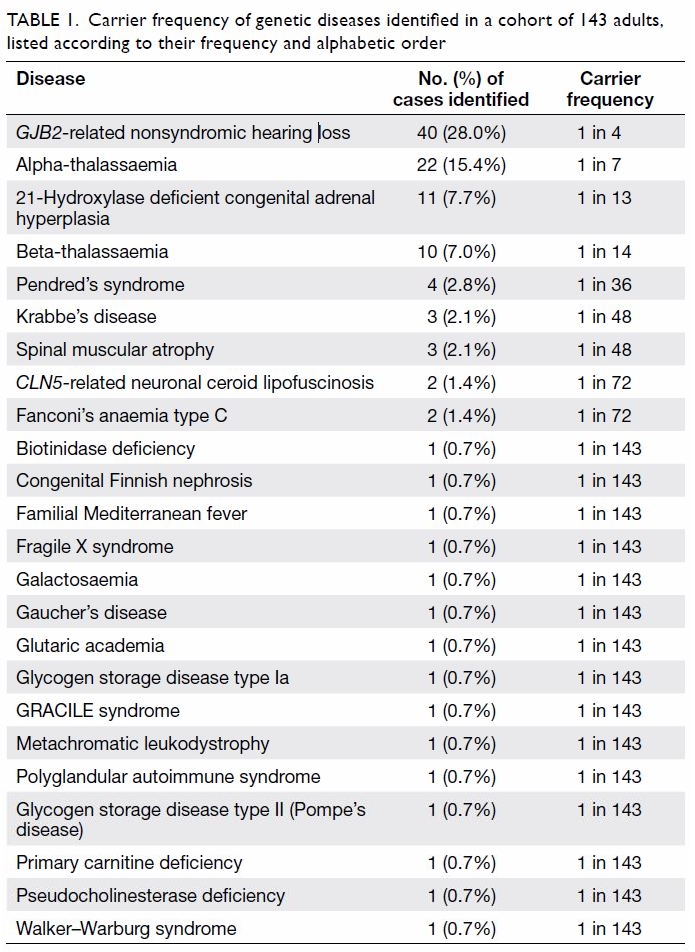
Table 1. Carrier frequency of genetic diseases identified in a cohort of 143 adults, listed according to their frequency and alphabetic order
Prevalence of carriers of various diseases
A total of 24 recessive diseases were identified in 84
(58.7%) of the 143 subjects. The data are summarised
in Table 1. The most common condition identified
was GJB2-related hearing loss (frequency: 1 in 4).
One subject was also found to be a homozygote for
the p.V37I mutation in the GJB2 gene. The subject
was aged 34 years and did not complain of hearing
impairment at the time of recruitment. Both alpha- and
beta-thalassaemia were prevalent in this cohort
(1 in 7 and 1 in 14, respectively), as shown in Table 1.
Eleven subjects (1 in 13) were identified as carriers
of the 21-hydroxylase deficient type of CAH. Four
subjects were heterozygous carriers of Pendred’s
syndrome (1 in 36), and three subjects were
heterozygous carriers for each of SMA and Krabbe’s
disease (1 in 48). Two carriers were identified for
both CLN5-related neuronal ceroid lipofuscinosis
and Fanconi’s anaemia type C, and one carrier was
identified for each of 15 other recessive conditions
(Table 1).
Multiple-disease carriers
The frequency of multiple-disease carriers is shown
in Table 2. Carrier status of at least two recessive
conditions was identified in 24 subjects (24/143,
16.8%) including thalassaemias and 11 subjects
(7.7%) excluding thalassaemias.
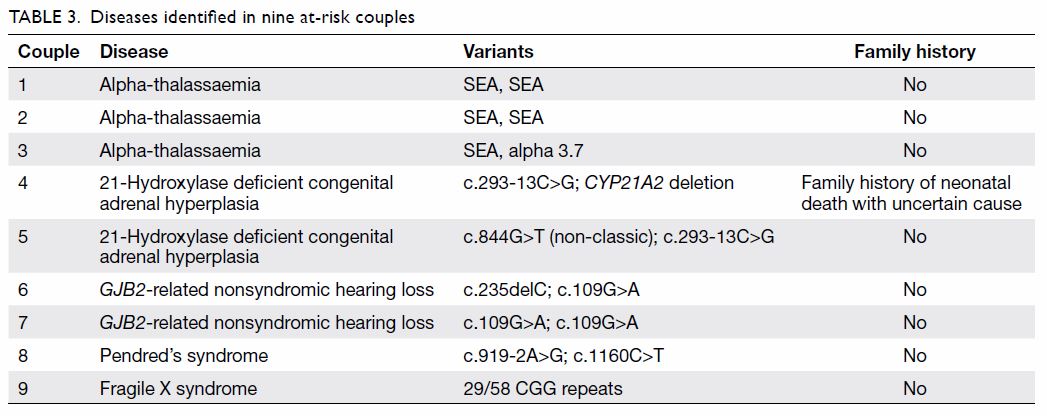
At-risk couples
One woman was a fragile X syndrome premutation
carrier, and 20 women had positive results for
carrier status, and their male partners were
sequentially tested. After integrating the sequential
testing results, we identified nine at-risk couples,
including three of alpha-thalassaemia, two of CAH,
two of GJB2-related hearing loss, one of Pendred’s
syndrome, and one of fragile X syndrome (Table 3).
The rate of at-risk couples was 12.0% (9/75) overall
and 8.0% (6/75) excluding thalassaemias.
Comparison between traditional screening
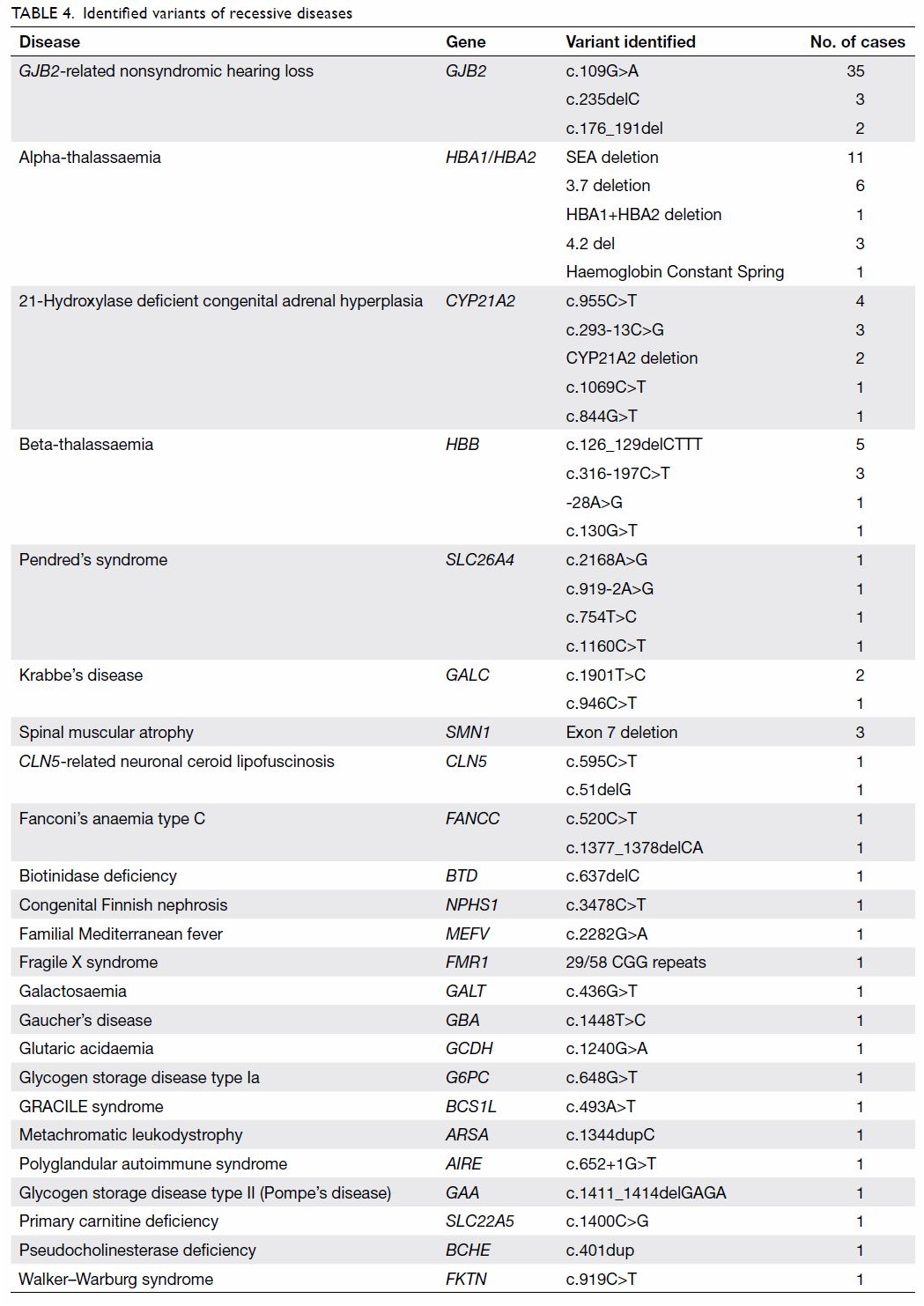
guidelines and next-generation sequencing
Forty three variants were identified by the NGS
panel (Table 4). Of the 43 variants, 29 (67.4%) were
not included in the ACMG or ACOG guidelines.9 11
Discussion
This study demonstrated the application of NGS to
investigate carrier frequency status of members of
the Chinese population in Hong Kong. The overall
positive yield of this expanded carrier screening
panel in our cohort was 58.7%. Not surprisingly,
both alpha- and beta-thalassaemia account for a
significant proportion of them. However, even after
excluding thalassaemias that could be screened by
mean corpuscular volume, the positive yield using
NGS was still as high as 47.6%, with 6 out of 75 at-risk
couples (8.0%) identified and potentially benefiting
from further pre-conception genetic counselling.
Although NGS has been increasingly used
for genetic carrier screening in Western countries
in recent years, there is a scarcity of data about the
carrier frequency of various recessive diseases
in the Chinese population. In 2013, Lazarin et al10
reported the carrier frequencies of a sample of
approximately 20 000 people from different ethnic
groups using a targeted mutation panel. East Asians
had the lowest carrier frequency (8.5%) compared
with Ashkenazi Jews (43.6%) or Caucasians (21%-32.6%). The most common genetic disease identified
among East Asians was GJB2-related hearing loss
(1 in 22), followed by beta-thalassaemia/sickle cell
disease (1 in 78) and SMA (1 in 85). However, the
assay used by Lazarin et al10 was partially based on
targeted genotyping, so carriers of variants other
than the included common mutations were not
detected. Thus, the reported carrier frequencies
are likely underestimated, particularly among East
Asians, as the common mutation panel was mainly
based on the Caucasian and Ashkenazi Jewish populations. In particular, alpha-thalassaemia and
CAH are not included in their panel.
Recently, Guo and Gregg15 investigated the carrier prevalence of 415 recessive diseases using
an exome sequencing database of approximately
120 000 samples. The consistent finding is that Ashkenazi Jews had the highest carrier frequency
(62.9%), followed by Caucasians, Africans, and
Hispanics; South and East Asians had the lowest
carrier frequency, but that frequency rose to 32.6%
with a more comprehensive panel. However, because
neither alpha-thalassaemia nor SMA was included
in the panel, the most common diseases for which
carrier status was found among East Asians were
autoimmune polyendocrinopathy syndrome type 1,
beta-thalassaemia, Usher’s syndrome type IIa, and
CAH. The carrier frequency of each of those diseases
was 1% to 2%. In 2018, Zhao et al16 reported >10 000
mainland Chinese couples in whom NGS was used
to screen for 11 recessive diseases. That study
showed a high carrier frequency of 27.49%, and 2.4%
of couples were carriers of the same genetic disease.
The authors found that the diseases with the highest
carrier frequencies were alpha-thalassaemia (15.1%),
beta-thalassaemia (4.8%), phenylketonuria (3.6%),
Wilson’s disease (2.0%), GJB2-related hearing loss
(1.7%), and Pendred’s syndrome (1.6%). However,
that study excluded SMA, CAH, and fragile X
syndrome.16 Our study’s findings are distinguished
from those of Lazarin et al,10 Guo and Gregg,15 and
Zhao et al16 in that we observed a much higher carrier
rate for GJB2-related hearing loss (28.0%), which is
consistent with our previous report (15.9%) using
target-enriched massively parallel sequencing.14 In
addition, we found higher carrier frequencies for
CAH (7.7%) and Pendred’s syndrome (2.8%). Our
study observed carrier frequency for SMA (2.1%)
is similar to that found in Western populations,17 18 19 20 21
indicating that SMA affects all ethnic groups.
One of the major limitations of our study was
the small sample size. More data are required before
we can draw precise conclusions regarding the carrier
frequency of individual recessive conditions
in the Chinese population. Second, patients in this
cohort were referred for subfertility or pre-pregnancy
counselling for genetic conditions, and give out of
this 123-patient cohort had a positive family history,
including thalassaemias, balanced translocation
carriers, family history of autism, neonatal death,
and previous pregnancy with structural abnormality.
Thus, some of the results might have been over-represented.
For example, one woman who presented
with subfertility was discovered to be a fragile X
permutation carrier, and this may have elevated
the carrier frequency of fragile X in our cohort of
123 women. In our previous study, in which we used
a robust polymerase chain reaction–based assay to
quantify fragile X CGG repeats for screening of 3000
low-risk Chinese pregnant women, the permutation
frequency was approximately 1 in 800.22 Another
couple in the present study had a previous baby with
neonatal death of unknown cause in Mainland China
and were found to be 21-hydroxylase deficient CAH carriers. Nonetheless, even after excluding these two
CAH cases, the CAH carrier frequency in our study
(1 in 16) remains high.
Currently, both the ACOG and ACMG
recommend carrier screening for SMA and cystic
fibrosis only in individuals of East Asian ethnicity.7 9
If those ethnic-based carrier screening strategies
advocated by the guidelines had been followed,
many carriers and all five carrier couples identified
in our cohort would have been missed. The results
of our pilot study suggest that recessive genetic
conditions may not be as uncommon as previously
thought. Many of the diseases identified in our
cohort are debilitating conditions that are associated
with progressive neurological derangement and
reduced life span, such as SMA, Krabbe’s disease,
and biotinidase deficiency. More importantly, some
conditions such as CAH may require intervention
during the early prenatal or early neonatal periods to
avoid irreversible complications. Hence, public and
professional awareness of expanded carrier screening
should be improved, and genetic counselling and
expanded carrier screening should be an option for
the Chinese population, especially in the setting of
subfertility clinics.
Yet, genetic carrier screening has not been
popular among the Chinese population or in Hong
Kong because of the high cost of the test and the
perceived low carrier rate in Chinese people. As the
cost for NGS has dropped recently, and our pilot
study demonstrated an overall high yield of 8.0% of
couples at risk of conceiving foetuses with genetic
diseases (even after excluding thalassaemias), further
studies of couples are warranted. Potential candidates
for expanded carrier screening in Hong Kong also
include couples in consanguineous marriages, which
are common in minor ethnic groups such as Pakistani
and Indian. A recent local study showed that they
had a higher prevalence of congenital abnormality
(10.5%), unexplained intrauterine foetal demise
(4.2%), and unexplained neonatal death (4.6%).23
In our cohort, NGS was used to analyse the
listed exons, as well as selected intergenic and intronic
regions, of the genes responsible for certain recessive
conditions. The high-throughput sequencing
technique was able to detect approximately 94% of
known clinically significant variants irrespective
of ethnicity. Of 43 variants identified using NGS,
29 (67.4%) were not included in the ACMG or ACOG
guidelines. Thus, our study demonstrated that the
NGS technique increased the detection rate of
carrier status for recessive conditions in the Chinese
population. Yet, further study with a larger sample
size should be conducted to study the prevalence of
carrier status, which conditions should be included,
and ethical issues related to carrier screening testing
such as reproductive options.
Conclusion
The observed carrier frequency in the Chinese
population was 58.7% overall (47.6% after excluding
thalassaemias) and was higher than previously
reported. Expanded carrier screening using NGS
should be provided to Chinese people to improve the
detection rate of carrier status and facilitate optimal
pregnancy planning.
Author contributions
All authors contributed to the concept or design of the
study, acquisition of data, analysis or interpretation of the
data, drafting of the manuscript, and critical revision of the
manuscript for important intellectual content.
All authors had full access to the data, contributed to the
study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
As an editor of the journal, JPW Chung was not involved in the peer review process. Other authors have disclosed no
conflicts of interest.
Funding/support
This research project was partially funded by the Liauw’s Family Reproductive Genomics Programme.
Ethics approval
This study obtained ethical approval from The Joint Chinese University of Hong Kongew Territories East Cluster
Clinical Research Ethics Committee (Ref CREC2019.138). All
participants gave informed consent before the study.
References
1. Edwards JG, Feldman G, Goldberg J, et al. Expanded
carrier screening in reproductive medicine-points to
consider: a joint statement of the American College of
Medical Genetics and Genomics, American College of
Obstetricians and Gynecologists, National Society of
Genetic Counselors, Perinatal Quality Foundation, and
Society for Maternal-Fetal Medicine. Obstet Gynecol
2015;125:653-62. Crossref
2. ACOG Committee on Obstetrics. ACOG Practice Bulletin
No. 78: hemoglobinopathies in pregnancy. Obstet Gynecol
2007;109:229-37. Crossref
3. Bajaj K, Gross SJ. Carrier screening: past, present and
future. J Clin Med 2014;3:1033-42. Crossref
4. ACOG Committee on Genetics. ACOG Committee
Opinion No. 442: preconception and prenatal carrier
screening for genetic diseases in individuals of Eastern
European Jewish descent. Obstet Gynecol 2009;114:950-3. Crossref
5. Eisenhower A, Suyemoto K, Lucchese F, Canenguez K.
“Which box should I check?”: examining standard check
box approaches to measuring race and ethnicity. Health
Serv Res 2014;49:1034-55. Crossref
6. American College of Obstetricians and Gynecologists Committee on Genetics. ACOG Committee Opinion No.
486: update on carrier screening for cystic fibrosis. Obstet
Gynecol 2011;117:1028-31. Crossref
7. Watson MS, Cutting GR, Desnick RJ, et al. Cystic fibrosis
population carrier screening: 2004 revision of American
College of Medical Genetics mutation panel. Genet Med
2004;6:387-91. Crossref
8. Prior TW, Professional Practice and Guidelines Committee.
Carrier screening for spinal muscular atrophy. Genet Med
2008;10:840-2. Crossref
9. Committee on Genetics. Committee Opinion No. 691:
carrier screening for genetic conditions. Obstet Gynecol
2017;129:e41-55. Crossref
10. Lazarin GA, Haque IS, Nazareth S, et al. An empirical
estimate of carrier frequencies for 400+ causal Mendelian
variants: results from an ethnically diverse clinical sample
of 23,453 individuals. Genet Med 2013;15:178-86. Crossref
11. Haque IS, Lazarin GA, Kang HP, Evans EA, Goldberg JD,
Wapner RJ. Modeled fetal risk of genetic diseases identified
by expanded carrier screening. JAMA 2016;316:734-42. Crossref
12. Feng Y, Ge X, Meng L, et al. The next generation of
population-based spinal muscular atrophy carrier
screening: comprehensive pan-ethnic SMN1 copy-number
and sequence variant analysis by massively parallel
sequencing. Genet Med 2017;19:936-44. Crossref
13. Shen J, Oza AM, Del Castillo I, et al. Consensus
interpretation of the p.Met34Thr and p.Val37Ile variants
in GJB2 by the ClinGen Hearing Loss Expert Panel. Genet
Med 2019;21:2442-52. Crossref
14. Choy KW, Cao Y, Lam ST, Lo FM, Morton CC, Leung TY.
Target-enriched massively parallel sequencing for genetic
diagnosis of hereditary hearing loss in patients with normal
array CGH result. Hong Kong Med J 2018;24 Suppl 3:11-4.
15. Guo MH, Gregg AR. Estimating yields of prenatal carrier
screening and implications for design of expanded carrier
screening panels. Genet Med 2019;21:1940-7. Crossref
16. Zhao S, Xiang J, Fan C, et al. Pilot study of expanded
carrier screening for 11 recessive diseases in China: results
from 10,476 ethnically diverse couples. Eur J Hum Genet
2019;27:254-62. Crossref
17. Li C, Geng Y, Zhu X, et al. The prevalence of spinal muscular
atrophy carrier in China: evidences from epidemiological
surveys. Medicine (Baltimore) 2020;99:e18975. Crossref
18. Evans M, McCarthy M, Moore R, Karbassi I, Alagia DP,
Lacbawan F. A comprehensive analysis of allele frequencies
from 476,930 spinal muscular atrophy test results [23M].
Obstet Gynecol 2019;133:147S. Crossref
19. Park JE, Yun S, Roh EY, Yoon JH, Shin S, Ki CS. Carrier
frequency of spinal muscular atrophy in a large-scale
Korean population. Ann Lab Med 2020;40:326-30. Crossref
20. Dejsuphong D, Taweewongsounton A, Khemthong P, et al.
Carrier frequency of spinal muscular atrophy in Thailand.
Neurol Sci 2019;40:1729-32. Crossref
21. Chen X, Sanchis-Juan A, French CE, et al. Spinal muscular
atrophy diagnosis and carrier screening from genome
sequencing data. Genet Med 2020;22:945-53. Crossref
22. Kwok YK, Wong KM, Lo FM, et al. Validation of a robust
PCR-based assay for quantifying fragile X CGG repeats.
Clin Chim Acta 2016;456:137-43. Crossref
23. Siong KH, Au Yeung SK, Leung TY. Parental consanguinity
in Hong Kong. Hong Kong Med J 2019;25:192-200. Crossref