Hong Kong Med J 2021 Feb;27(1):7–17 | Epub 27 Aug 2020
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Clinical and radiological characteristics of
COVID-19: a multicentre, retrospective,
observational study
Y Wang, MD1 # †;S Luo, MD2 #; CS Zhou, BS2 #; ZQ Wen, MD3 #; W Chen, MD4,5; W Chen, MD6; WH Liao, MD7; J Liu, MD7; Y Yang, MD9; JC Shi, MD10; SD Liu, MD10; F Xia, MS2; ZH Yan, MD5; X Lu, PhD11; T Chen, MD12; F Yan, PhD11; B Zhang, MD1 †; DY Zhang, MD9 †; ZY Sun, MD2 †
1 Department of Radiology, The Affiliated Nanjing Drum Tower Hospital of Nanjing University Medical School, Nanjing, Jiangsu, China
2 Department of Medical Imaging, Jinling Hospital, Medical School of Nanjing University, Nanjing, Jiangsu, China
3 Department of Outpatient, Jinling Hospital, Medical School of Nanjing University, Nanjing, Jiangsu, China
4 Department of Radiology, Jinling Hospital, Southern Medical University, Nanjing, Jiangsu, China
5 Department of Radiology, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China
6 Department of Medical Imaging, Taihe Hospital, Shiyan, Hubei, China
7 Department of Medical Imaging, Xiangya Hospital of Central South University, Changsha, Hunan, China
8 Department of Medical Imaging, The Second Xiangya Hospital of Central South University, Changsha, Hunan, China
9 Department of Medical Imaging, Wuhan First Hospital, Wuhan, Hubei, China
10 Department of Infectious Disease, Wenzhou Central Hospital, Wenzhou, Zhejiang, China
11 State Key Laboratory of Natural Medicines, Research Center of Biostatistics and Computational Pharmacy, China Pharmaceutical University, Nanjing, Jiangsu, China
12 Medical School of Nanjing University, Nanjing, Jiangsu, China
# Y Wang, S Luo, CS Zhou, and ZQ Wen equally contributed to this work
† Y Wang, ZY Sun, DY Zhang, and B Zhang equally contributed to this work
Corresponding author: Dr Y Wang (wangzhang227@163.com)
Abstract
Background: Multicentre cohort investigations of
patients with coronavirus disease 2019 (COVID-19)
have been limited. We investigated the clinical and
chest computed tomography characteristics of
patients with COVID-19 at the peak of the epidemic
from multiple centres in China.
Methods: We retrospectively analysed the
epidemiologic, clinical, laboratory, and radiological
characteristics of 189 patients with confirmed
COVID-19 who were admitted to seven hospitals
in four Chinese provinces from 18 January 2020 to
3 February 2020.
Results: The mean patient age was 44 years and
52.9% were men; 186/189 had ≥1 co-existing medical
condition. Fever, cough, fatigue, myalgia, diarrhoea,
and headache were common symptoms at onset;
hypertension was the most common co-morbidity.
Common clinical signs included dyspnoea, hypoxia,
leukopenia, lymphocytopenia, and neutropenia;
most lesions exhibited subpleural distribution. The
most common radiological manifestation was mixed
ground-glass opacity with consolidation (mGGO-C);
most patients had grid-like shadows and some
showed paving stones. Patients with hypertension,
dyspnoea, or hypoxia exhibited more severe lobe
involvement and diffusely distributed lesions.
Patients in severely affected areas exhibited higher
body temperature; more fatigue and dyspnoea;
and more manifestations of multiple lesions, lobe
involvement, and mGGO-C. During the Wuhan
lockdown period, cough, nausea, and dyspnoea
were alleviated in patients with newly confirmed
COVID-19; lobe involvement was also improved.
Conclusions: Among patients with COVID-19
hospitalised at the peak of the epidemic in
China, fever, cough, and dyspnoea were the main
symptoms at initial diagnosis, accompanied by
lymphocytopenia and hypoxaemia. Patients with
severe disease showed more severe lobe involvement
and diffuse pulmonary lesion distribution.
New knowledge added by this study
- Among patients with coronavirus disease 2019 (COVID-19) hospitalised during the peak of the epidemic in China, common clinical signs included dyspnoea, hypoxia, leukopenia, lymphocytopenia, and neutropenia; most lesions exhibited subpleural distribution. The most common radiological manifestation was mixed ground-glass opacity with consolidation.
- Patients with hypertension were likely to exhibit hypoxaemia; furthermore, their lung lobes were severely involved and lesions were significantly diffusely distributed.
- All patients with severe disease showed mixed ground-glass opacity with consolidation; paving stones and grid-like shadows were significantly associated with the presence of mixed ground-glass opacity with consolidation.
- Patients in severely affected areas demonstrated slightly higher body temperature, more frequent fatigue, and more frequent dyspnoea. After implementation of the ‘Wuhan lockdown’ policy, cough, nausea, and dyspnoea were significantly alleviated in patients with newly confirmed COVID-19.
- Mixed ground-glass opacity with consolidation, paving stones, and grid-like shadows might serve as comprehensive indicators of disease severity in patients with COVID-19.
- Radiological examinations should be used as the primary screening method in this epidemic because of their efficiency, instead of the current approach of body temperature checks and reverse-transcriptase polymerase chain reaction assays.
- Patients with hypertension require close clinical monitoring, as they are more likely to exhibit hypoxaemia. Proactive interventions (eg, positive pressure ventilation) are needed to enhance blood oxygen concentration.
- As COVID-19 progresses, patients begin to develop immunosuppression; lymphopenia may therefore be a key factor related to disease severity and mortality in these patients.
Introduction
Pneumonia caused by severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2), known as
coronavirus disease 2019 (COVID-19), has become a pandemic.1 2 3 By 1 March 2020, a total of 87 137
cases were confirmed globally, including 79 968
in China and 7169 distributed across 58 countries
outside China. On 23 January 2020, the Chinese
Central Government imposed a lockdown in
Wuhan and other cities in Hubei Province to isolate
the epicentre of the outbreak; to the best of our
knowledge, the ‘Wuhan lockdown’ represents the
first lockdown of a major city in modern history.4
Subsequently, compulsory policies have been
established, such as suspension of public gatherings
and the requirement to wear masks.5 In addition to
non-medical interventions, the scientific community
is responding to this challenge by working to
understand and control the disease.6 7 8 9 10 11 However,
there have been limited studies regarding the clinical
and radiological features of patients with COVID-19,
based on multicentre, multiprovincial cohorts at the
peak of the epidemic.
In this context, we retrospectively analysed
189 patients with laboratory-confirmed COVID-19,
using data collected from seven hospitals in four
provinces from 18 January 2020 to 3 February 2020.
We compared clinical features, laboratory tests, and
chest computed tomography (CT) image findings of
these patients with respect to time (ie, before and
after Wuhan lockdown) and space (ie, in and outside
of Wuhan epidemic area); we investigated potential
associations between CT findings and laboratory
data. Our findings may help further understand the
epidemiological characteristics of COVID-19 and
improve the public health response to the epidemic;
they can be used as a reference for implementing
control measures in other regions or countries with
increasing numbers of patients with COVID-19.
Methods
Study design and participants
Data regarding 189 patients were obtained from the
following seven hospitals: Taihe Hospital (n=59),
Xiangya Hospital of Central South University
(n=21), The Second Xiangya Hospital of Central
South University (n=9), Wenzhou Hospital (n=76),
Jinling Hospital (n=1), Nanjing Drum Tower
Hospital (n=12), and Wuhan Hospital (n=11).
Wenzhou is the prefecture-level city with the most
confirmed cases outside Hubei Province. Symptom
onset time was recorded from 31 December 2019
to 2 February 2020; patients were hospitalised
from 18 January 2020 to 3 February 2020, with
final follow-up for this report on 4 February 2020.
Patients with suspected COVID-19 were admitted
and quarantined, then diagnosed with COVID-19 in
accordance with the ‘Novel Coronavirus Pneumonia
Prevention and Control Program’ (sixth edition)—a
patient was confirmed to have COVID-19 based on
high throughput sequencing or real-time reverse-transcriptase
polymerase chain reaction (RT-PCR)
assays of nasal and pharyngeal swab specimens.12
Two target genes—open reading frame 1ab (ORF1ab)
and nucleocapsid protein (N)—were simultaneously
amplified and tested in real-time RT-PCR
assays. Target 1 (ORF1ab) comprised forward
primer 5’-CCCTGTGGGTTTTACACTTAA-3’,
reverse primer 5’-ACGATTGTGCATCAGCTGA-3’,
and the probe
5’-VIC-CCGTCTGCGGTATGTGGAAAGGTTATGG-BHQ1-3’.
Target 2 (N) comprised forward primer
5’-GGGGAACTTCTCCTGCTAGAAT-3’, reverse
primer 5’-CAGACATTTTGCTCTCAAGCTG-3’,
and the probe 5’-FAMTTGCTGCTGCTTGACAGATT-TAMRA-3’.
Real-time RT-PCR assays were conducted using
a SARS-CoV-2 nucleic acid detection kit, in
accordance with the manufacturer’s protocol
(Shanghai BioGerm Medical Technology Company,
Shanghai, China).
Data collection
Epidemiological, clinical, laboratory, and
radiological characteristics were obtained from
electronic medical records by using data collection
forms. Date of disease onset was defined as the
day when symptoms were noticed. The number of
days between symptom onset and date of the first
positive test was recorded. Fever was defined as an
axillary temperature of ≥37.5°C. Hypoxaemia was
defined as 94% oxygen saturation, in accordance
with respiratory department criteria. Major CT
features were described using standard international
nomenclature, defined by the Fleischner Society
glossary and peer-reviewed literature regarding viral
pneumonia. Degree of COVID-19 severity at the
time of admission was defined as mild, moderate,
severe, or critical, using preliminary diagnostic
guidance from the National Health Commission of
the People’s Republic of China. Disease was further
separated into non-severe (ie, mild and moderate)
and severe (ie, severe and critical) classifications
for simplicity. Direct exposure history was defined
as patient confirmation of a direct visit to Wuhan,
China, during a particular period; close exposure
history was defined as close patient contact with
an individual who had confirmed or suspected
COVID-19. To analyse spatiotemporal differences,
patients were divided into different categories
according to the start date for the Wuhan lockdown (ie, 23 January 2020) for temporal analysis or heavy
epidemic province classification (eg, Hubei and
Zhejiang provinces13) for spatial analysis.
Statistical analyses
Continuous variables were described as the mean
(±standard deviation); categorical variables were
expressed as frequency (%). All statistical analyses
were conducted with R (version 3.6.2; https://www.r-project.org/), using Fisher’s exact test for categorical
data and a two-sample Mann-Whitney test or
Student’s t test for continuous data (as appropriate).
Correlations were measured by Pearson’s correlation
coefficient (ρ). Co-morbidities, signs, and symptoms
that appeared in more than 10% of the patients were
regarded as common co-existing medical conditions.
For unadjusted comparisons, two-sided P values
<0.05 were considered statistically significant. The
analyses were not adjusted for multiple comparisons;
thus, given the potential for type I error, the findings
should be interpreted as exploratory and descriptive.
Results
Presenting characteristics
The present study included 189 patients with
confirmed COVID-19 from four provinces in
China, including 70 (37.0%) from Hubei (11 [5.8%]
from Wuhan), 30 (15.9%) from Hunan, 76 (40.2%)
from Zhejiang, and 13 (6.9%) from Jiangsu. Dates
of confirmed infection by RT-PCR ranged from
18 January 2020 to 3 February 2020. The mean date of
disease onset was 21 January 2020; the mean date of
confirmed infection by RT-PCR was 28 January 2020.
The mean duration between symptomology onset
and first positive test was 6±5 days. Patients in
severely affected areas (eg, Hubei and Zhejiang
provinces) showed symptoms earlier than patients
in other areas (Fig 1).
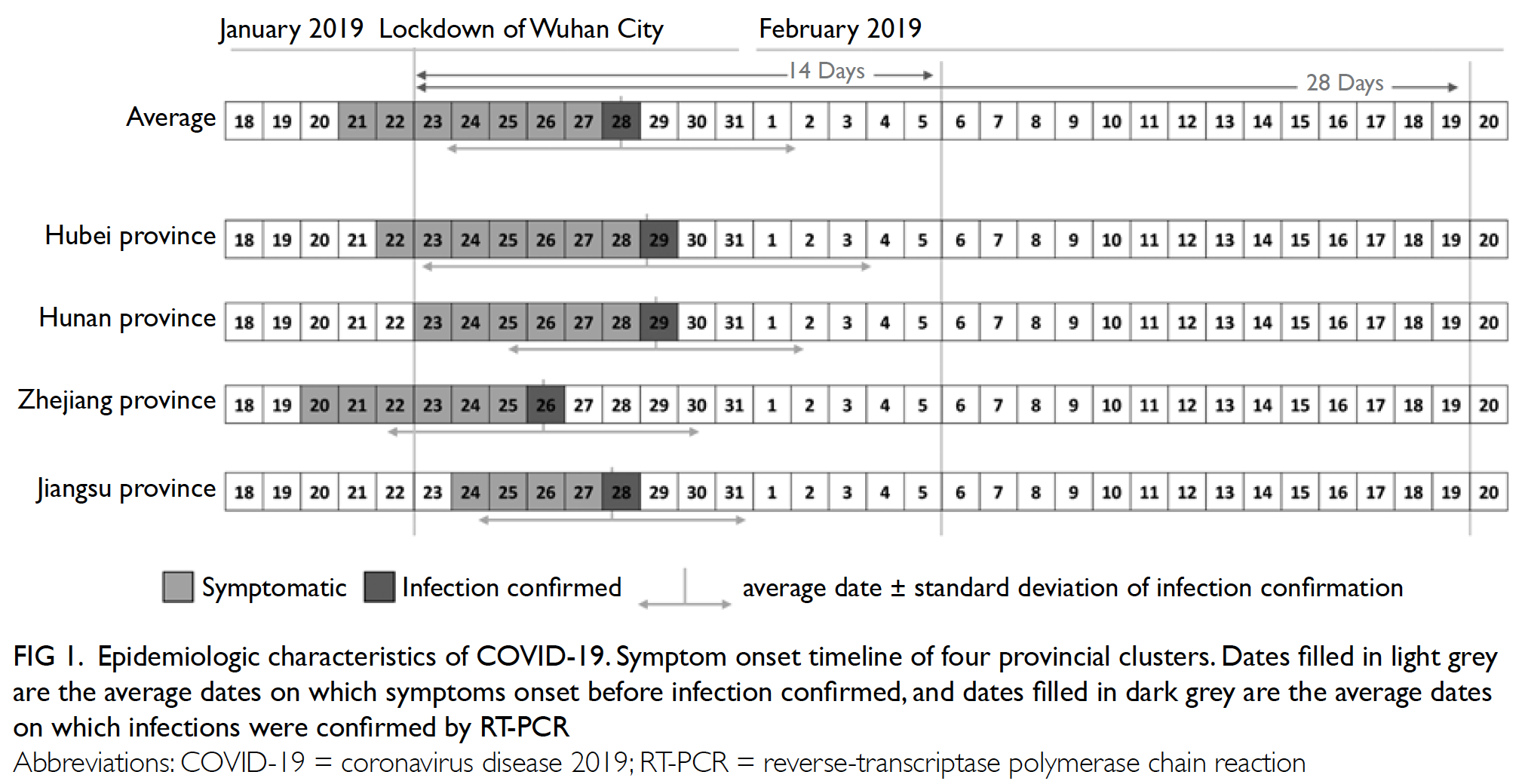
Figure 1. Epidemiologic characteristics of COVID-19. Symptom onset timeline of four provincial clusters. Dates filled in light grey are the average dates on which symptoms onset before infection confirmed, and dates filled in dark grey are the average dates on which infections were confirmed by RT-PCR
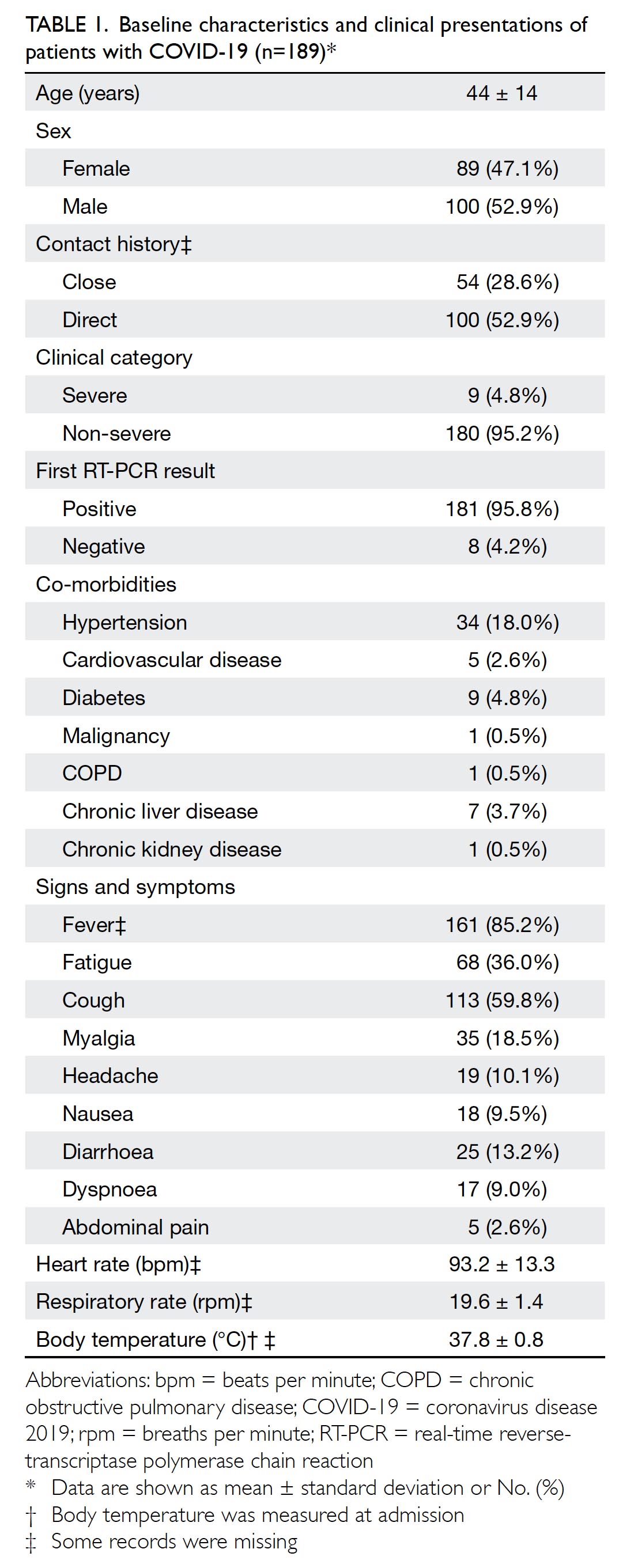
The overall characteristics of included patients
are summarised in Table 1. The mean age was
44±14 years (range, 17-92 years); 100 patients
(52.9%) were men. Of the 189 patients, 181 (95.8%)
exhibited positive findings for COVID-19 in the
initial RT-PCR assay; 186 patients (98.4%) had
≥1 co-existing medical condition. Fever (161 [86.1%];
two missing records), cough (113 [59.8%]), fatigue
(68 [36.0%]), myalgia (35 [18.5%]), diarrhoea
(25 [13.2%]), and headache (19 [10.1%]) were the
most common symptoms at onset; hypertension
(34 [18.0%]) was the most common co-morbidity.
Less common co-morbidities included chronic
obstructive pulmonary disease, chronic kidney
disease, and malignancy (one patient each). Most
patients (180 [95.2%]) had non-severe disease;
patients with severe disease tended to be older
(P=0.067) and had significantly greater breathing
frequency (P=0.009) than patients with non-severe disease (online supplementary Appendix 1).
Notably, fever was the primary symptom indicative
of COVID-19 in patients with suspected disease
during the epidemic; however, in our cohort, fever
was independent of other imaging findings (data not
shown).
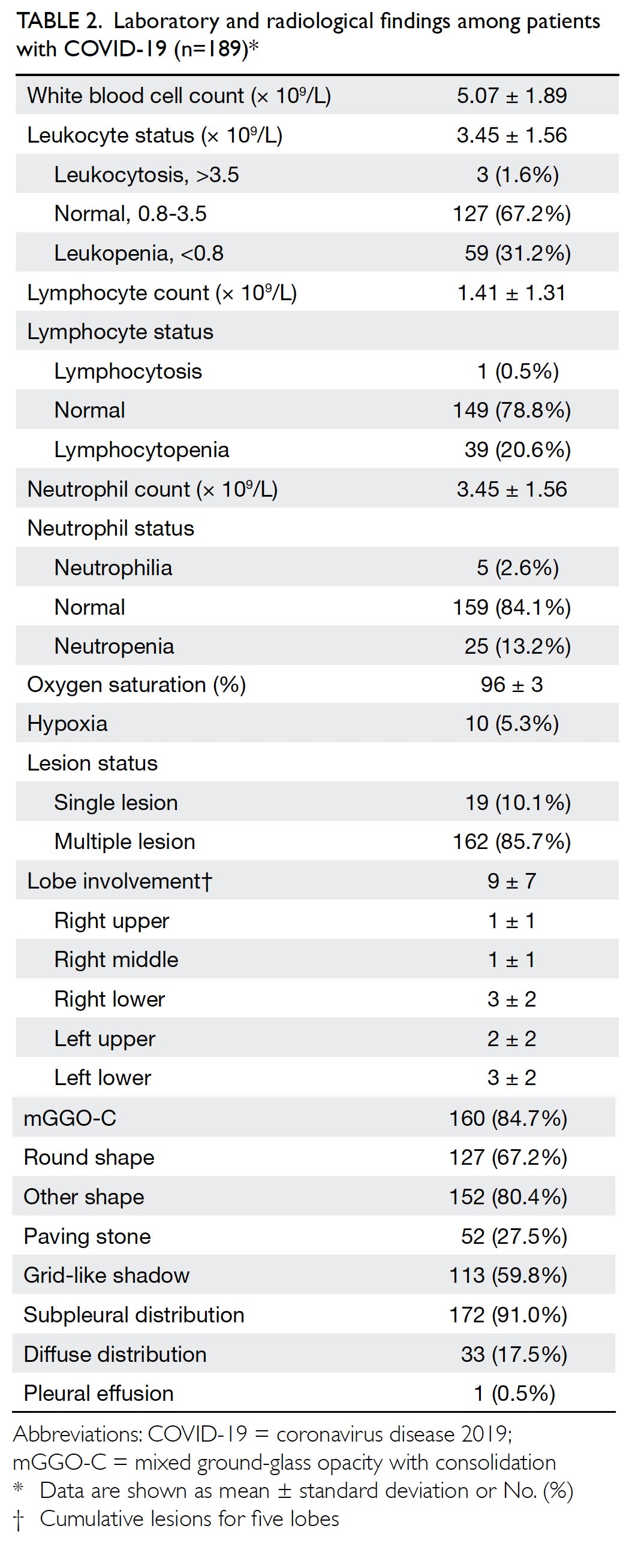
On admission, 10 patients (5.3%) presented
with hypoxaemia at the time of initial diagnosis; leukopenia was present in 31.2% of the patients,
lymphocytopenia was present in 20.6%, and
neutropenia was present in 13.2%. Patients with
critical disease had more laboratory abnormalities
than those with severe disease, including
lymphocytopenia (44.4% vs 19.6%; P=0.09) and
hypoxaemia (55.6% vs 2.8%; P<0.001). Approximately
91.0% of lesions exhibited subpleural distribution;
17.5% of lesions were diffusely distributed. The most
common patterns on chest CT were mixed ground-glass
opacity with consolidation (mGGO-C, 84.7%);
59.8% of patients had grid-like shadows and 27.5%
of patients exhibited radiological manifestations of
typical paving stones (Fig 2). Patients with severe
disease showed more frequent involvement of
multiple lobes (all P<0.05) and more frequent diffuse
distribution (P=0.008), compared with patients who
exhibited non-severe disease (online supplementary Appendix 1).
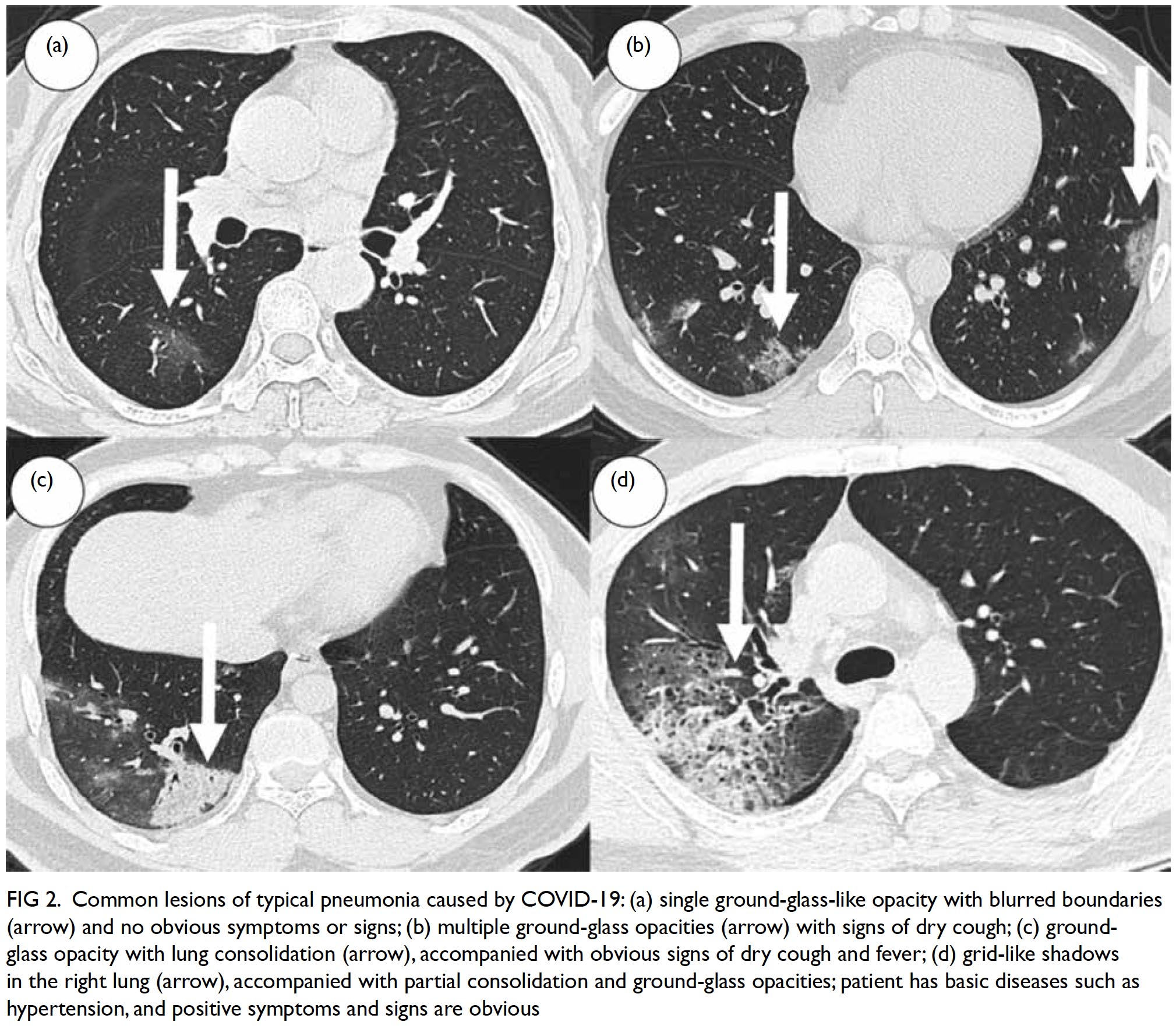
Figure 2. Common lesions of typical pneumonia caused by COVID-19: (a) single ground-glass-like opacity with blurred boundaries (arrow) and no obvious symptoms or signs; (b) multiple ground-glass opacities (arrow) with signs of dry cough; (c) groundglass opacity with lung consolidation (arrow), accompanied with obvious signs of dry cough and fever; (d) grid-like shadows in the right lung (arrow), accompanied with partial consolidation and ground-glass opacities; patient has basic diseases such as hypertension, and positive symptoms and signs are obvious
Severity of hypoxia and dyspnoea
To evaluate the severity of respiratory damage caused by COVID-19, 26 patients (13.8%) who presented
with subjective dyspnoea or objective hypoxia
were selected for detailed analysis (Table 2). These
26 patients were generally older (50±16 years;
P=0.013) and showed more diverse clinical symptoms
(eg, cough [80.8%; P=0.033], fatigue [53.8%;
P=0.068], nausea [30.8%; P<0.001], diarrhoea [34.6%;
P=0.002], and abdominal pain [11.5%; P=0.017]),
co-morbidities (eg, hypertension [38.5%; P=0.008]),
and haematological abnormalities (eg, lymphocytopenia
[38.5%; P=0.051]), compared with patients who did
not exhibit dyspnoea or hypoxia. The radiological
manifestations in these patients were not optimistic
because all patients demonstrated multiple lesions
(100%; P=0.123) and mGGO-C (100%; P=0.361);
many patients had diffusely distributed lesions
(42.3%; P=0.001) and grid-like shadows (80.8%;
P=0.033) [Table 3]. Among nine patients with
severe disease, seven (77.8%) had varying degrees of
hypoxia and dyspnoea. Additionally, patients with
haematological abnormalities (especially leukopenia
or lymphocytopenia) showed more severe lobe involvement and tended to show diffusely distributed
pulmonary lesions (online supplementary Appendix 2). Specifically, the white blood cell count
was significantly negatively correlated with the
numbers of lesions in left upper (ρ=-0.18, P=0.012),
left lower (ρ=-0.23, P=0.002), and right lower lobes
(ρ=-0.19, P=0.009).
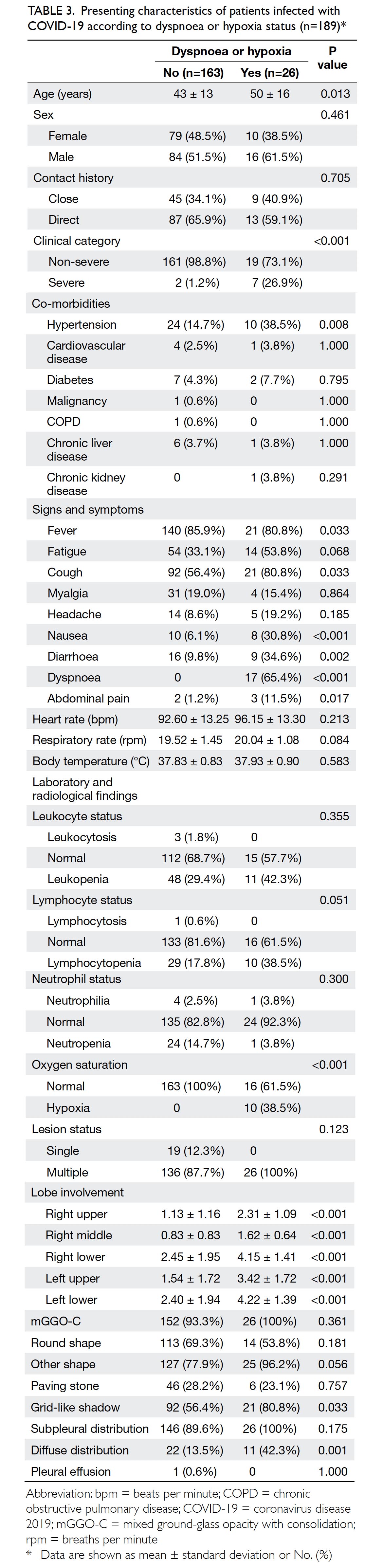
Table 3. Presenting characteristics of patients infected with COVID-19 according to dyspnoea or hypoxia status (n=189)
Subgroup analysis of patients with
hypertension
Although there is no evidence that patients with
hypertension are more susceptible to COVID-19,
18.0% of patients with confirmed disease exhibited
hypertension, which was the most common clinical
co-morbidity in our cohort. These patients were
likely to exhibit hypoxaemia (14.7%; P=0.022);
furthermore, their lung lobes were severely
involved (all P<0.05) and lesions were significantly
diffusely distributed (35.3%; P=0.006). Therefore,
these patients require close clinical monitoring
(Table 4).
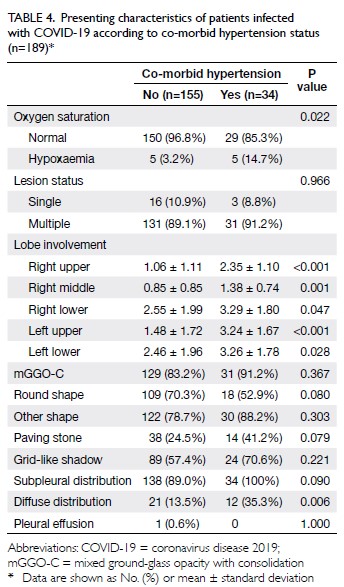
Table 4. Presenting characteristics of patients infected with COVID-19 according to co-morbid hypertension status (n=189)
Co-occurrence of unfavourable radiological
manifestations
In this cohort, all patients with severe disease showed
mGGO-C. Paving stones, a sign of the inflammatory
absorption period, and grid-like shadows, a sign of
interstitial lung lesions, were significantly associated
with the presence of mGGO-C (both P<0.05);
thus, these radiological findings might serve as
comprehensive indicators of disease severity in
patients with COVID-19 (online supplementary Appendix 3). Additionally, these patients also
showed unfavourable imaging findings, including
multiple lesions and severe lung lobe involvement
(all P≤0.001).
Spatial and temporal differences
To further investigate the spatiotemporal differences
among patients with COVID-19, we compared
clinical, laboratory, and radiological characteristics
of patients with respect to the start date of the
Wuhan lockdown, as well as heavy epidemic
province classification status (online supplementary Appendices 4 and 5). We found that patients in
severely affected areas (eg, Hubei and Zhejiang
provinces) demonstrated slightly higher body
temperature (mean: 37.9℃ vs 37.6℃; P=0.070),
more frequent fatigue (39.7% vs 23.3%, P=0.072),
and more frequent dyspnoea (11.6% vs 0, P=0.041),
compared with patients in other areas. Imaging
findings showed more manifestations of multiple
lesions (92.9% vs 78.0%, P=0.031), more severe
lobe involvement, and more frequent radiological
manifestations of mGGO-C (87.7% vs 74.4%,
P=0.060). Additionally, after implementation of the
‘Wuhan lockdown’ policy, the symptoms of cough
(51.0% vs 70.1%, P=0.012), nausea (3.9% vs 16.1%,
P=0.010), and dyspnoea (2.0% vs 17.2%, P=0.001)
were significantly alleviated in patients with newly
confirmed COVID-19; lung lobe involvement was
also dramatically improved, compared with patients
who had been diagnosed prior to the start date of the
lockdown.
Discussion
This study assessed the epidemiological, clinical,
laboratory, and imaging characteristics of 189 patients
with confirmed COVID-19 from multiple hospitals
and provinces; it also included spatiotemporal
analysis of disease in these patients. As expected,
there were more male patients than female patients
in our cohort; fever, cough, and dyspnoea were the
main symptoms at the time of initial diagnosis,
accompanied by lymphocytopenia, hypoxaemia, and
other haematological abnormalities. Furthermore,
patients with severe disease showed significantly
more severe lobe involvement and diffuse
distribution of pulmonary lesions, consistent with
the findings in previous studies.6 11 12 13 14 Reductions in
the numbers of white blood cells or lymphocytes
are closely associated with lobe involvement and
diffuse distribution, such that a large number of
inflammatory cells is consumed at pulmonary lesions
in a short period; this finding is consistent with past
pathological findings in patients with COVID-1915—interstitial mononuclear inflammatory infiltrates, dominated by lymphocytes, have been observed
in both lungs; multinucleated syncytial cells with
atypical enlarged pneumocytes (characterised by
large nuclei, amphophilic granular cytoplasm, and
prominent nucleoli) were identified in intra-alveolar
spaces, which constituted a viral cytopathy-like
change. Additionally, lymphopenia is a common
laboratory finding in patients with COVID-19.
A serological study16 and a pathological result15
demonstrated that patients’ interleukin-6 levels
increased during the course of the disease, whereas
the levels of CD4+ T cells, CD8+ T cells, and natural
killer cells decreased. These findings imply that, as
the disease progresses, patients begin to develop
immunosuppression; lymphopenia may therefore be
a key factor related to disease severity and mortality
in patients with COVID-19.
Another important finding in this study
was that patients with hypertension were likely to
exhibit hypoxaemia, accompanied by unfavourable
radiological manifestations; this was presumably
because the patients included in this study were mostly middle-aged or elderly people. The reported
prevalence of hypertension in China was 23.2%
in adults,17 which was slightly higher than the
prevalence in our cohort. In general, older people
are more susceptible to COVID-19 and more likely
to experience severe disease, compared with people
younger than 50 years of age, because older people
exhibit greater numbers of health conditions and
co-morbidities. Notably, the zinc metallopeptidase
angiotensin-converting enzyme 2 (ACE2)18 19—a
negative regulator of the angiotensin system which
affects heart function, hypertension, and diabetes—has been identified as a key receptor for SARS-CoV-2
in humans. Angiotensin-converting enzyme 2
protects against acute lung injury in several animal
models of acute respiratory distress syndrome,
which indicates that the renin-angiotensin system
may play a critical role in the pathogenesis of acute
lung injury. Thus, enhancement of ACE2 activity
might be a novel approach for the treatment of
acute lung failure in several diseases. Angiotensin-converting
enzyme 2 receptors are widely expressed
in nasal mucosa, bronchus, lung, heart, oesophagus,
kidney, stomach, bladder, and ileum; importantly,
the entrance of SARS-CoV-2 into cells mainly
occurs through binding to ACE2. Thus, unlike other
β-coronaviruses, SARS-CoV-2 replication is not
limited to the upper respiratory mucosa epithelium
(eg, nasal cavity and pharynx); it also occurs in the
digestive tract and other organs, which partially
explains the non-respiratory symptoms (eg,
diarrhoea, liver damage, and kidney damage).20
Multiple affected organs cause diverse clinical
manifestations and large individual differences,
which lead to complex conditions. Accordingly,
patients with a history of hypertension should
receive closer monitoring.
Respiratory system infections end in
respiratory failure or multiple organ failure.21
Similar to previous reports of patients with severe
acute respiratory syndrome (SARS), some patients
in the present study experienced dyspnoea and
hypoxaemia during the course of COVID-19 (online supplementary Appendix 6). Notably, our patients
showed greater numbers of clinical symptoms and
unfavourable imaging findings. Pathologically, SARS
mainly causes the formation of hyaline membranes,
which result in large numbers of inflammatory
exudates into the alveolar cavity, as well as patchy
haemorrhage and focal necrosis; these changes lead
to respiratory failure and extremely high mortality.
In contrast, our patients with COVID-19 generally
exhibited mGGO-C as the main imaging feature,
which causes airway obstruction without obvious
hyaline membrane formation; thus, ventilator
support can be used to improve patient prognosis.
We presume that the presence of early imaging
findings indicates that proactive interventions (eg, positive pressure ventilation) are needed to enhance
blood oxygen concentration.
Fever, the most common symptom at the first
visit and the most commonly used indicator for
COVID-19, showed no significant relationship with
radiological findings in the present study, which
implies that patients may show no abnormalities
(eg, changes in body temperature) when obvious
lesions form in the lungs. Furthermore, a recent
study22 demonstrated that the sensitivity of RT-PCR
for confirmation of COVID-19 is lower than the
sensitivity of chest imaging scans, which also suggests
that radiological examinations should be used as the
primary screening method in this epidemic because
of their efficiency, instead of the current approach of
body temperature checks and RT-PCR assays.
Overall, the spatial distribution of the epidemic
demonstrated here is consistent with the official
statistics.23 The distribution of disease incidence
had a clear relationship with population mobility. In
particular, cities surrounding Wuhan (throughout
Hubei Province) reported the vast majority of cases,
followed by Wenzhou (Zhejiang Province), which has
a large floating population from Wuhan. Wan et al24
and Wrapp et al25 showed that SARS-CoV-2 is
more infectious than SARS-CoV. Imported cases
were most common in the early period of the
epidemic; symptoms then began to appear among
individuals who had been in contact with the first
group of infected individuals, which contributed to
a rapid increase in the number of infections. The
symptoms of fatigue and dyspnoea were alleviated
outside severely affected areas, which implied
reduction of virus potency during intergenerational
transmission and early admission to hospitals. In the
present study, we used the date of disease onset for
analysis of affected patients. Patients in Hubei and
Zhejiang provinces showed symptoms earlier and
were confirmed to have COVID-19 an average of
6 days later, compared with patients in other areas;
these findings coincide with the reported 14-day
incubation period.26 Gradually, clinical symptoms
and chest CT findings were alleviated in patients
with newly confirmed COVID-19 after the beginning
of the Wuhan lockdown; these changes also implied
reduction of virus potency during intergenerational
transmission.
In general, the radiological manifestations
of COVID-19 are similar to those of SARS and
Middle East respiratory syndrome (MERS), but
pleural effusion is rare in patients with COVID-19
(online supplementary Appendix 6). In two previous
studies,27 28 the proportions of patients with SARS
and MERS who had pleural effusions were 25%
(4/16) and 14.5% (8/55), whereas only one patient
with COVID-19 (0.5%) had pleural effusions in our
cohort. Current studies indicate that the binding
forces between the SARS-CoV-2 S protein and human ACE2 are similar to (or stronger than) those
between the SARS-CoV S protein and its receptor.25
Given the state of the epidemic, SARS-CoV-2 is
highly infectious; its basic reproduction number
(R0) is considerably greater than that of either SARS
or MERS.29 The World Health Organization reported
that the R0 of SARS-CoV-2 ranged from 1.4 to 2.5,
whereas a study in China indicated an R0 of 3.3 to
5.530 and a study in the United States estimated an
R0 of 3.77 (95% confidence interval, 3.51-4.05).31
The findings of our multicentre retrospective study
demonstrate that current measures have affected
the early epidemiological pattern (ie, rapid increase)
because R0 is decreasing each day in China; however,
considering the complexity of influencing factors,
further evaluations and predictions are needed.
The majority of patients with COVID-19 exhibit
non-severe disease, which is an essential source of
infection and a ‘blind spot’ for public health efforts;
therefore, CT findings such as infiltration, nodules,
and consolidation should be identified during early
diagnosis. Notably, flu season is approaching rapidly;
there is a need for attention to epidemiological
history and condition monitoring, as well as efforts
to block routes of transmission as quickly as possible.
We acknowledge some limitations in this
study. First, the cohort size was relatively small,
and data were not collected equally from each
included province, which may have led to bias
in the conclusions. Second, documentation was
incomplete for some patients, given the variations in
electronic database structures among participating
sites and the urgent timeline for data extraction.
Missing data included contact history, heart rate,
respiratory rate, and body temperature. Because of
the small numbers of patients for whom these data
were missing, the main conclusions of this study
were presumably unaffected. Third, the sizes and
densities of mGGO-C were not compared among
patients; thus, analysis of relationships between
these characteristics and COVID-19 progression
warrants investigation.
Interpretation
Clinical and imaging features were compared among
patients with COVID-19 at the peak of epidemic
in China. The findings suggest that mGGO-C,
paving stones, and grid-like shadows might serve as
comprehensive indicators of disease severity in these
patients. Furthermore, radiological examinations
may be useful as the primary screening method in
this epidemic because of their efficiency, in contrast
to the current approach of body temperature checks
and RT-PCR assays.
Overall spatiotemporal trends were also
evaluated retrospectively in this study. Patients in
severely affected areas demonstrated slightly higher body temperature, more frequent fatigue, and
more frequent dyspnoea. After implementation of
the ‘Wuhan lockdown’ policy, cough, nausea, and
dyspnoea were significantly alleviated in patients
with newly confirmed COVID-19. These data
indicate that the preventive measures adopted by
China’s Central Government may be appropriate for
planning efforts in other regions or countries with
increasing numbers of infected patients.
Author contributions
Concept or design: Y Wang, F Yan, B Zhang, DY Zhang, and ZY Sun.
Acquisition of data: ZQ Wen, W Chen, W Chen, WH Liao, J Liu, Y Yang, JC Shi, SD Liu, F Xia, and ZH Yan.
Analysis or interpretation of data: X Lu, T Chen, and Y Wang.
Drafting of the manuscript: Y Wang, S Luo, CS Zhou, X Lu, and T Chen.
Critical revision of the manuscript for important intellectual content: Y Wang, B Zhang, DY Zhang, and Z Sun.
Acquisition of data: ZQ Wen, W Chen, W Chen, WH Liao, J Liu, Y Yang, JC Shi, SD Liu, F Xia, and ZH Yan.
Analysis or interpretation of data: X Lu, T Chen, and Y Wang.
Drafting of the manuscript: Y Wang, S Luo, CS Zhou, X Lu, and T Chen.
Critical revision of the manuscript for important intellectual content: Y Wang, B Zhang, DY Zhang, and Z Sun.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
The authors declare no competing interests.
Acknowledgement
We thank Prof Guangming Lu (Department of Medical
Imaging, Jinling Hospital, Medical School of Nanjing
University, Nanjing, Jiangsu, China) for his coordination
during the cross-centre data collection process. We also thank
all hospital staff for their efforts in collecting the information
used in this study; all patients who consented to inclusion
of their data in the analysis; and all medical staff involved in
patient care.
Funding/support
This work was supported by the National Natural Science
Foundation of China (81720108022, 91649116, 81571040,
81973145), the Social Development Project of Science and
Technology in Jiangsu Province (BE2016605, BE201707), the
National Key R&D Program of China (2017YFC0112801), the
Key Medical Talents of Jiangsu Province, the ‘13th Five-Year’
Health Promotion Project of Jiangsu Province (B.Z.2016-2020),
the Jiangsu Provincial Key Medical Discipline (Laboratory)
(ZDXKA2016020), the Project of the Sixth Peak of Talented
People (WSN-138, BZ), the China Postdoctoral Science
Foundation (2019M651805), the “Double First-Class”
University project (CPU2018GY09), and Nanjing Health
and Family Planning Commission (YKK17089). The funders
had no role in study design, data collection, data analysis,
interpretation, or writing of the report.
Ethics approval
This study adhered to the tenets of the Declaration of Helsinki
and was approved by the ethics committees of the seven
hospitals (Taihe Hospital, Xiangya Hospital of Central South
University, The Second Xiangya Hospital of Central South
University, Wenzhou Hospital, Jinling Hospital, Nanjing Drum Tower Hospital, and Wuhan Hospital) with a unified
approval number [M202003050028] led by Nanjing Drum
Tower Hospital; a waiver of informed consent was granted
because the study involved patients with emerging infectious
diseases.
References
1. Silverstein WK, Stroud L, Cleghorn GE, Leis JA. First
imported case of 2019 novel coronavirus in Canada,
presenting as mild pneumonia. Lancet 2020;395:734. Crossref
2. Holshue ML, DeBolt C, Lindquist S, et al. First case of
2019 novel coronavirus in the United States. N Engl J Med
2020;382:929-36. Crossref
3. Pongpirul WA, Pongpirul K, Ratnarathon AC,
Prasithsirikul W. Journey of a Thai taxi driver and novel
coronavirus. N Engl J Med 2020;382:1067-8. Crossref
4. Lee J. Wuhan lockdown ‘unprecedented’, shows
commitment to contain virus: WHO representative in
China. 23 Jan 2020. Available from: https://www.reuters.com/article/us-china-health-who-idUSKBN1ZM1G9.
Accessed 23 Jan 2020.
5. Chen S, Yang J, Yang W, Wang C, Bärnighausen T. COVID-19
control in China during mass population movements at
New Year. Lancet 2020;395:764-6. Crossref
6. Chen N, Zhou M, Dong X, et al. Epidemiological and
clinical characteristics of 99 cases of 2019 novel coronavirus
pneumonia in Wuhan, China: a descriptive study. Lancet
2020;395:507-13. Crossref
7. Kanne JP. Chest CT findings in 2019 novel coronavirus
(2019-nCoV) infections from Wuhan, China: key points
for the radiologist. Radiology 2020;295:16-7. Crossref
8. Li Q, Guan X, Wu P, et al. Early transmission dynamics in
Wuhan, China, of novel coronavirus–infected pneumonia.
N Engl J Med 2020;382:1199-207. Crossref
9. Ng MY, Lee EY, Yang J, et al. Imaging profile of the
COVID-19 infection: radiologic findings and literature
review. Radiol Cardiothorac Imaging 2020;2:e200034. Crossref
10. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138
hospitalized patients with 2019 novel coronavirus–infected
pneumonia in Wuhan, China. JAMA 2020;323:1061-9. Crossref
11. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics
of coronavirus disease 2019 in China. N Engl J Med
2020;382:1708-20. Crossref
12. World Health Organization. Coronavirus disease
(COVID-19) technical guidance publications. Laboratory
testing for 2019 novel coronavirus (2019-nCOV) in
suspected human cases. 2020. Available from: https://www.who.int/publications/i/item/10665-331501. Accessed 19
Mar 2020.
13. Wu Z, McGoogan JM. Characteristics of and important
lessons from the coronavirus disease 2019 (COVID-19)
outbreak in China: summary of a report of 72 314 cases
from the Chinese Center for Disease Control and
Prevention. JAMA 2020 Feb 24. Epub ahead of print. Crossref
14. Clinical findings in a group of patients infected with
the 2019 novel coronavirus (SARS-Cov-2) outside of
Wuhan, China: retrospective case series [editorial]. BMJ
2020;368:m792. Crossref
15. Xu Z, Shi L, Wang Y, et al. Pathological findings of
COVID-19 associated with acute respiratory distress
syndrome. Lancet Respir Med 2020;8:420-2. Crossref
16. Wan S, Yi Q, Fan S, et al. Characteristics of lymphocyte
subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus
pneumonia (NCP). medRxiv [Preprint] 12 Feb 2020.
Available from: https://doi.org/10.1101/2020.02.10.20021832. Accessed 19 Mar 2020. Crossref
17. Chen WW, Gao RL, Liu LS, et al. China cardiovascular diseases report 2015: A summary. J Geriatr Cardiol
2017;14:1-10.
18. Kuba K, Imai Y, Penninger JM. Angiotensin-converting
enzyme 2 in lung diseases. Curr Opin Pharmacol
2006;6:271-6. Crossref
19. Turner AJ, Hiscox JA, Hooper NM. ACE2: from
vasopeptidase to SARS virus receptor. Trends Pharmacol
Sci 2004;25:291-4. Crossref
20. Huang C, Wang Y, Li X, et al. Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China.
Lancet 2020;395:497-506. Crossref
21. Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel
coronavirus associated with severe acute respiratory
syndrome. N Engl J Med 2003;348:1953-66. Crossref
22. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT
for COVID-19: comparison to RT-PCR. Radiology
2020;296:E115-7. Crossref
23. National Health Commission of the People’s Republic
of China. Update on COVID-19 epidemic as of 24:00 on
1 March 2020 [in Chinese]. 2020. Available from: http://www.nhc.gov.cn/xcs/yqtb/202003/5819f3e13ff6413ba05fd
b45b55b66ba.shtml. Accessed 2 Mar 2020.
24. Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor
recognition by novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS
Coronavirus. J Virol 2020;94:e00127-20. Crossref
25. Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure
of the 2019-nCoV spike in the prefusion conformation.
Science 2020;367:1260-3. Crossref
26. Diagnosis and Treatment Protocol for Novel Coronavirus
Pneumonia (Trial Version 7). 2020. Available from: http://www.shliangshi.com/newsshow_825.html. Accessed 3
Mar 2020.
27. Hsieh SC, Chan WP, Chien JC, et al. Radiographic
appearance and clinical outcome correlates in 26 patients
with severe acute respiratory syndrome. AJR Am J
Roentgenol 2004;182:1119-22. Crossref
28. Das KM, Lee EY, Al Jawder SE, et al. Acute Middle East
Respiratory Syndrome Coronavirus: temporal lung
changes observed on the chest radiographs of 55 patients.
AJR Am J Roentgenol 2015;205:W267-74. Crossref
29. Paules CI, Marston HD, Fauci AS. Coronavirus infections—more than just the common cold. JAMA. 2020 Jan 23. Epub
ahead of print. Crossref
30. Zhao S, Lin Q, Ran J, et al. Preliminary estimation of
the basic reproduction number of novel coronavirus
(2019-nCoV) in China, from 2019 to 2020: A data-driven
analysis in the early phase of the outbreak. Int J Infect Dis
2020;92:214-7. Crossref
31. Kim JY, Choe PG, Oh Y, et al. The first case of 2019 novel
coronavirus pneumonia imported into Korea from Wuhan,
China: implication for infection prevention and control
measures. J Korean Med Sci 2020;35:e61. Crossref