Hong Kong Med J 2021 Feb;27(1):27–34 | Epub 4 Feb 2021
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Evaluation of bronchial challenge test results for use in assessment of paediatric eczema: a retrospective series
KL Hon, MB, BS, MD1; Abraham HY Ng, MB, ChB2; Chrystal CC Chan, MB, ChB2; Prisca XY Ho, MB, ChB2; Emma PM Tsoi, MB, ChB2; Kathy YC Tsang, MPhil, BSc1Fanny W Ko, MB, ChB, MD3; TF Leung, MB, ChB, MD1
1 Department of Paediatrics, The Chinese University of Hong Kong, Hong Kong
2 Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong
3 Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong
Corresponding author: Dr KL Hon (ehon@hotmail.com)
Abstract
Background: Atopic dermatitis (AD), asthma, and
allergic rhinitis are associated diseases involved in
the atopic march. The bronchial challenge test (BCT)
is a tool that evaluates airway hyperresponsiveness
in patients with asthma. This study aimed to evaluate
whether a positive BCT result is useful in assessment
of paediatric AD.
Methods: This retrospective case series included
284 patients with AD who had BCT results. Clinical
information and laboratory parameters were
reviewed, including AD severity (using the SCORing
Atopic Dermatitis [SCORAD]), skin hydration, and
transepidermal water loss.
Results: Of the 284 patients who had BCT, 106 had
positive BCT results and 178 had negative BCT
results. A positive BCT result was associated with
a history of asthma (P<0.0005), sibling with asthma
(P=0.048), serum immunoglobulin E (P=0.045),
eosinophil count (P=0.017), and sensitisation to
food allergens in the skin prick test (P=0.027). There
was no association between a positive BCT result
and personal allergic rhinitis, parental atopy, sibling
allergic rhinitis or AD, skin prick response to dust
mites, objective SCORAD score, skin hydration,
transepidermal water loss, exposure to smoking,
incense burning, cat or dog ownership, or AD
treatment aspects (eg, food avoidance and traditional Chinese medicine). Logistic regression showed
significant associations of a positive BCT result
with a history of asthma (adjusted odds ratio=4.05;
95% confidence interval=1.92-8.55; P<0.0005)
and sibling atopy (adjusted odds ratio=2.25;
95% confidence interval=1.03-4.92; P=0.042).
Conclusions: In patients with paediatric AD,
a positive BCT result was independently and
positively associated with personal history of asthma
and sibling history of atopy, but not with any other
clinical parameters.
New knowledge added by this study
- In paediatric patients with atopic dermatitis (AD), a positive bronchial challenge test (BCT) result was significantly associated with a history of asthma (adjusted odds ratio [aOR]=4.05; 95% confidence interval [CI]=1.92-8.55; P<0.0005) and sibling atopy (aOR=2.25; 95% CI=1.03-4.92; P=0.042).
- BCT results were independently and positively associated with a personal history of asthma and sibling history of asthma, but not with any other clinical parameters of AD.
- BCT may have limited usefulness in patients with AD beyond the prediction of asthma prevalence or risk.
- Alternatives to the BCT should be sought with greater clinical predictive strength.
Introduction
Atopic dermatitis (AD) or eczema, asthma, and
allergic rhinitis (AR) are associated diseases involved
in the atopic march.1 2 3 Many children with AD develop
asthma and/or AR when they reach adulthood.
There are a number of clinical and laboratory tools
to evaluate atopic status in patients with AD. The bronchial challenge test (BCT) is an important tool
that evaluates airway hyperresponsiveness in patients
with asthma. Responsive patients develop acute
contraction of smooth muscles lining the bronchi,
resulting in sudden narrowing and obstruction of the
airway. The extent of airway narrowing can increase
during periods of exacerbation and decrease during treatment with anti-inflammatory drugs.4 The
inhalation of histamine or methacholine produces
direct airway responses. Histamine maximises
bronchial obstruction by directly activating H1
histamine receptors during inhalation challenge.
It also stimulates nasal and mucus secretion,
promotes vasodilation, and increases vascular
permeability. Methacholine is a synthetic derivative
of the acetylcholine neurotransmitter, which directly
stimulates M3 muscarinic receptors on airway
smooth muscles to induce bronchoconstriction.4 A low inhaled dose could trigger a high degree of
airway hyperresponsiveness in patients with asthma.
In BCT, methacholine and histamine stimulate
an increase in cyclic guanosine monophosphate level
and a decrease in cyclic adenosine monophosphate
level, thus contributing to smooth muscle
contraction.5 Immediate bronchoconstriction
reactions can begin immediately after challenge;
the peak is within approximately 30 minutes. The
effect is typically reversible within 1.5 to 2 hours
with the aid of bronchodilators. This is regarded
as a type 1 hypersensitivity reaction mediated
by immunoglobulin E (IgE), which is present in
patients with hypersensitivity pneumonitis, asthma,
and other atopic diseases. Immunoglobulin E has
a specific role in the induction of many allergic
reactions as evidenced by its high serum level in
patients with allergic diseases and those with atopic
diseases.6 7
Distinct dosages are used for the inhalation
of methacholine and histamine, but the inhalation
challenge procedures are identical.8 During the
test, a diluent is provided via nebulisation for five
inhalations, followed by nebulisation of the test
compound at low concentrations. A spirometry
test is conducted after each dilution to assess the
patient’s pulmonary function. A reduction in forced
expiratory volume in 1 second (FEV1) of ≥20%
signifies a positive response and the end of the test.
A bronchodilator is then provided to counteract the
effects of the test compound. In subsequent tests,
the dose that provokes the desirable ≥20% reduction
of FEV1 is employed.5
There are several contra-indications for
the BCT. Patients with reduced lung function as
evidenced by a low FEV1 level in baseline spirometry
may be predisposed to a greater risk of serious
adverse events.9 A prior bronchodilator FEV1 <60%
predicted or FEV1 <75% predicted during a single
high stimulus (eg, exercise) are relative contra-indications.10 Airway obstruction in baseline
spirometry supplemented with clinical features of
asthma is sufficient for diagnosis and the BCT is
unnecessary. Furthermore, an inability to follow
the instructions of the spirometry test undermines
the BCT quality.11 Individuals with a history of
cardiovascular problems, increased intracranial
pressure, or recent eye surgery may experience
enhanced cardiovascular stress as a consequence
of bronchoconstriction during the BCT and should
not be subjected to this test.4 10 In general, patients
with asthma respond to methacholine and histamine
even at low concentrations, such that 84% and 73%
react to the above compounds, respectively.12
This study aimed to investigate whether
personal characteristics, history of allergen
exposure, skin prick test results, clinical assessment
scores, laboratory parameters, and personal or
family histories of atopic diseases are associated
with a positive BCT result in paediatric patients with
AD. The result is useful in counselling parents and
patients with AD and risks of asthma in the family.
Methods
This retrospective case series included patients with
AD who were treated at the paediatric dermatology
clinic of a university hospital from January 2000 to
November 2017; patients who had undergone the
BCT were retrospectively selected for analysis. All
selected patients were Chinese and aged >8 years (the
minimum age at which patients can complete the
BCT in our hospital pulmonology laboratory).4 11 13
Data concerning the following clinical and personal
characteristics were obtained from the patients’
medical records: AD onset age, history of other
atopy (ie, AR and atopic asthma), history of atopy (ie,
AD, atopic asthma and AR) in parents and siblings, potential allergen exposure (ie, pet ownership and
tobacco smoking), and AD treatment history (ie,
allergen avoidance and use of traditional Chinese
medicine). Clinical laboratory parameters obtained
from the electronic patient record system included
BCT results, highest serum IgE level, highest
eosinophil counts (absolute and relative), and skin
prick test results for allergic response to dust mite,
cockroach, dogs, cats, and food allergens.
Atopic dermatitis severity was clinically scored
by the SCORing Atopic Dermatitis (SCORAD),
skin hydration, and transepidermal water loss
(TEWL) during follow-up visits.14 The SCORAD is
a mathematically derived score that considers the
extent, severity, and subjective symptoms of AD.15
The skin hydration and TEWL were measured using
Courage and Khazaka equipment, which indirectly
measures the density gradient of water evaporation
from the skin by two pairs of sensors that determine
the temperature and relative humidity, respectively.16
The IBM SPSS Statistics (Windows version 20)
was used to conduct all statistical analysis. Student’s
t test was conducted to compare continuous
variables between groups; these data were expressed
as mean ± standard deviation. The Chi squared test
(or Fisher’s exact test) was conducted to compare
categorical variables between groups; these data
were expressed as number (%). A P value of <0.05
was considered statistically significant. Backward
binary logistic regression analysis was then used
to analyse the data. The variable with the highest
P value was removed at each step until the final
equation included only variables with P<0.05.
The New Territories East Cluster and The
Chinese University of Hong Kong ethics committee
approved this retrospective study.
Results
Overall patient characteristics
In total, 579 patients with AD were included in
this study; 284 had confirmed BCT results. Patient
follow-up data were censored as of November 2017 to
February 2018. Of the 284 patients with BCT results,
106 had a positive result (BCT-positive group), while
178 had a negative result (BCT-negative group).
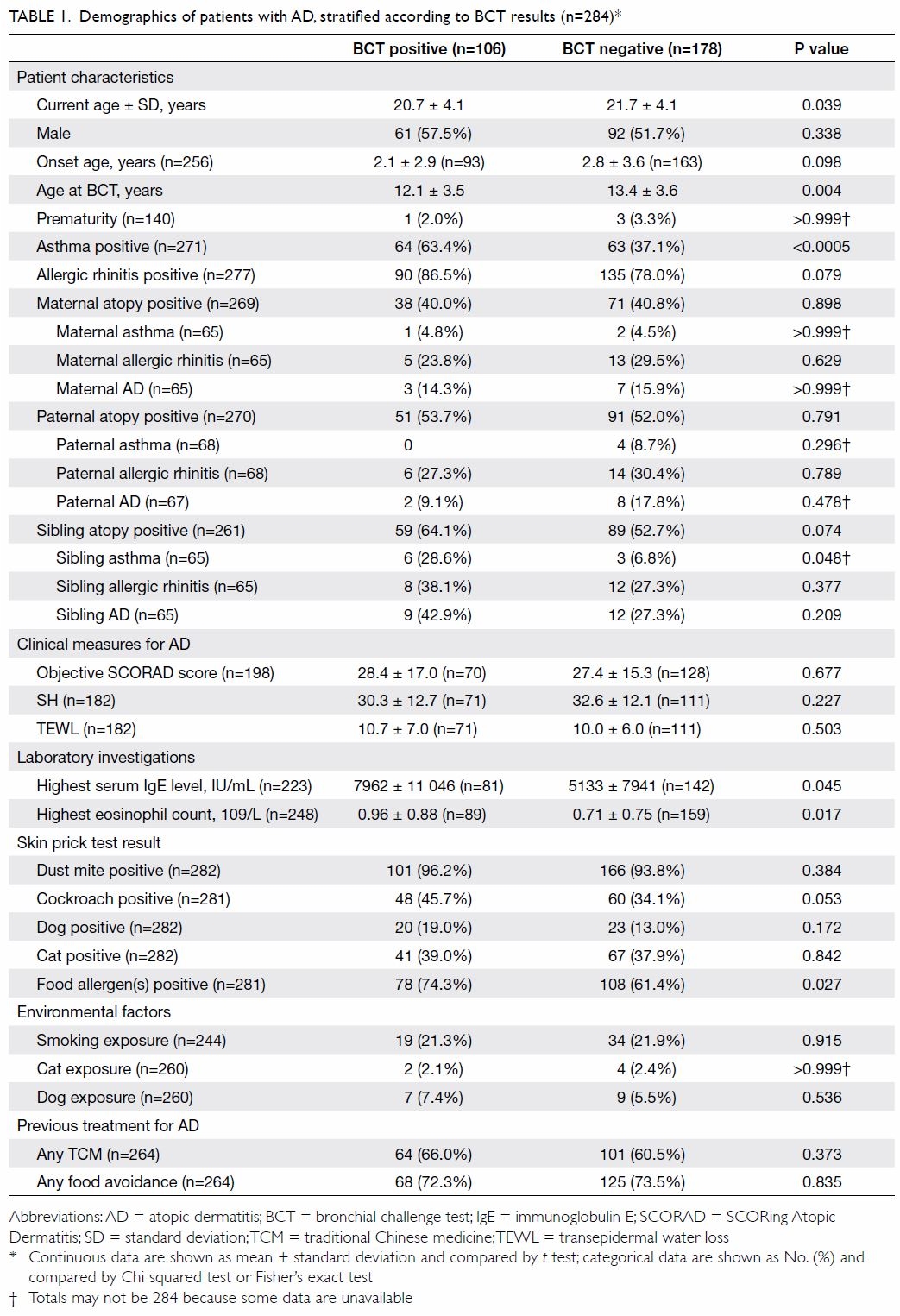
There were significant differences in current age
(P=0.039) and the age at BCT performance (P=0.004)
between groups (Table 1).
Personal and family histories of atopic
diseases
A positive BCT result was associated with personal
history of asthma (P<0.0005) and a sibling with
asthma, compared with patients with a negative
BCT result (28.6% vs 6.8%; P=0.048) [Table 1].
There were no significant associations
between BCT results and personal AR, maternal atopy (specifically, asthma, AR, AD), paternal atopy (specifically asthma, AR, AD), siblings’ AR,
and siblings’ AD. Moreover, BCT results were not
associated with a history of skin prick response to
allergens, including dust mites (Table 1).
Clinical measures, laboratory results,
environmental factors, and previous treatment
There were no significant associations between
BCT results and clinical measures of AD severity,
in terms of objective SCORAD score, SH, or TEWL.
Results of BCT were associated with markers of
allergic reactions, including (highest) serum IgE
(P=0.045), highest eosinophil count (P=0.017), and
sensitisation to food allergens in skin prick test
(P=0.027). However, they were not associated with
other allergens tested in the skin prick panel, such
as aeroallergens from dust mites, cockroaches, dogs,
or cats (Table 1). Results of BCT had no significant
association with previous exposure to potential
environmental irritants, including smoking in the
household, incense burning, cat ownership, or dog
ownership. There were no significant associations
between BCT results and history of AD treatment,
including food avoidance and traditional Chinese
medicine usage (Table 1).
Regression analysis
Of the 284 patients with AD and confirmed BCT
results, 142 patients had all relevant data available.
Backward binary logistic regression (n=142) showed
significant associations between a positive BCT
result and a personal history of asthma (adjusted
odds ratio [aOR]=4.05; 95% confidence interval
[CI]=1.92-8.55; P<0.0005) and sibling atopy
(aOR=2.25; 95% CI=1.03-4.92; P=0.042). However, it
did not show associations with AD severity, young
age at AD onset, personal history of AR, aeroallergen
(including dust mite, cockroach, cat and dog hair)
and food allergen sensitisation, parental history
of atopy, or highest serum IgE level and blood
eosinophil count (Table 2).
Discussion
Atopic dermatitis and asthma
In our study, backward binary logistic regression
analysis showed that a positive BCT result was
independently and positively associated with
a personal history of asthma in patients with
AD. The Global Initiative for Asthma, a medical
guidelines organisation, has established a standard
for the diagnosis of asthma, which recommends
the documentation of variable expiratory airflow
limitation. Although asthma is principally a clinical
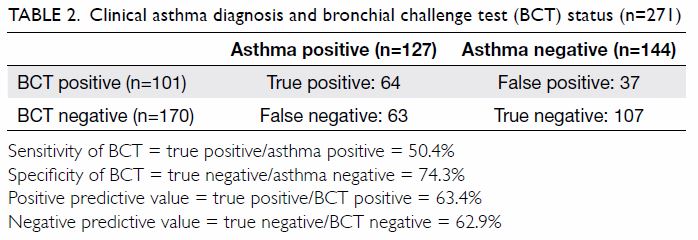
diagnosis, the BCT can aid in this assessment.17 The
sensitivity of the BCT in complementing asthma diagnosis is generally believed to approach 100% if
a cut point is set at 8 mg/mL or 16 mg/mL, using a
non-deep inhalation method.18 Cockcroft18 showed
that when the PC20 (ie, histamine provocative
concentration causing a 20% drop in FEV1) cut-off
was set at ≤8 mg/mL, all individuals with current
symptomatic asthma could be identified in a random
population with a sensitivity of nearly 100%.1
However, a recent study indicated that the
prevalence of a positive BCT result in children with
asthma is approximately 70%.19 This is attributed
to the potential effects of several factors associated
with a positive BCT result, especially using the
methacholine challenge test; these factors include
AR, respiratory infections, and chronic respiratory
conditions (eg, bronchitis and chronic obstructive
pulmonary disease).19 Despite its limitations,
including that the test can only be used in older
children, the BCT remains a useful tool for the
diagnosis of asthma (Table 2). Clinical usage of
the BCT in the diagnosis of asthma is based on its
high sensitivity and negative predictive value, but
a positive BCT result itself is not confirmatory for
asthma.17 20
In this study of 284 patients with BCT results,
the aOR of a positive BCT result for asthma in
patients with AD was 4.05, implying that patients
with AD who have a positive BCT result are fourfold
more likely to have asthma. This is consistent with
the findings of a 2015 meta-analysis that included
31 studies conducted in 102 countries; the risk
ratio of AD to other two atopic disorders, including
AR and asthma, was 4.24 (95% CI=3.75-4.79). The
results demonstrated a clear relationship between
the skin and the airways.21 Riiser et al22 followed
530 children who completed the BCT at the age of
10 years and underwent structured reviews and
clinical examinations at the age of 16 years; they
examined the predictability of BCT results for
active asthma in adolescence. The investigators
concluded that the presence of bronchial
hyperreactivity (BHR), especially severe BHR, was
a significant risk factor and that provocative dose
20 resulting in 20% decrease in FEV1 in a positive
test alone explained 10% of the variation in active
asthma.22 Another study showed similar findings,
in that airway hyperresponsiveness independently
predicted asthma symptoms in childhood (aOR=2.6;
95% CI=1.8-3.7).23 Our results concurred with those
of the above studies, indicating that BHR could
develop before active asthma symptoms appear and
that BCT might indeed predict asthma incidence in
children with AD.
Sibling history of atopy
Atopy is known to have a strong hereditary
component. A family study of 188 Caucasian patients
and their family members demonstrated a twofold increase in risk of developing AD and atopy with
each additional first-degree relative who exhibited
atopy.24 Furthermore, a maternal history of atopic
disease was associated with an elevated IgE level
among infants. For maternal asthma, this association
was only evident in infant girls.25 Hong Kong has a
relatively high prevalence of single-child families;
however, among patients with siblings, positive
relationships with sibling atopy have been found.1 2
In our study, among 106 patients with a positive
BCT result, rates of maternal, paternal, and sibling
atopy were 40.0%, 53.7%, and 64.1%, respectively.
A positive BCT result was independently and
positively associated with sibling atopy (aOR=2.25;
95% CI=1.03-4.92; P=0.042), which includes
asthma, AR, and/or AD. However, no associations
with parental atopy were found. Patients with
positive BCT results are presumably more likely to
have siblings with atopy, in that 64.1% of patients
with positive BCT results had siblings with atopy,
compared with 52.7% of patients with negative
BCT results. The most likely explanations of the
association with siblings involve genetic heredity
and similar epigenetic factors in childhood. Siblings
of patients with atopy are predisposed to an
inherited tendency to developing IgE antibodies to
specific allergens, with subsequent hypersensitivity
reactions. Several twin studies were conducted to
demonstrate the roles of genetic and environmental
factors on atopy. A multivariate genetic analysis
with a total of 575 twin participants revealed that
atopic conditions were associated with genetic and
environmental factors; it also showed that different
phenotypic conditions shared common genetic
backgrounds.26 Family and twin studies have shown
that genetic inheritance plays an important role in
the development of atopy; they identified 79 genes
associated with asthma or atopy in more than one
population.27 Despite genetic involvement, atopic
conditions are highly heterogeneous and involve
complex epigenetic factors. The atopic state is
presumably related to a pre-existing genotype that
is activated by environmental factors.28 Both the
factors themselves and duration of exposure are
important. Several epigenetic factors with an impact
on atopy have been established; these can be further
subcategorised into prenatal, infancy, childhood, and adulthood types.29 Affected children and their
siblings are exposed to common environmental
factors in their childhood, such as tobacco smoke,
allergic sensitisation, infections, and diet.
There was minimal information concerning
birth order and family size in the present study,
although these factors may have substantial impacts
on allergic disease susceptibility. Asthma prevalence
is reportedly inversely related to family size in
families with ≥4 children.30 An inverse relationship
between birth order and asthma risk has also
been suggested. Compared with younger siblings,
stronger associations with older siblings with asthma
have been demonstrated.30 However, family size was
not specified in those studies; thus, there is a need
to determine whether family size or birth order
exerts a greater impact upon the risk of atopy. The
mechanism by which younger children are protected
against atopy is unknown. One possible explanation
is the hygiene hypothesis, which suggests that
children with less exposure to pathogens and
other microorganisms in early childhood are more
susceptible to allergic diseases. An implication is
that the attempt to create dust-free and pathogen-free
clean environment leads to increased atopy
prevalence. The presence of older siblings and larger
family size is presumed to protect children from
atopy through greater exposure in early childhood,
thus modulating their immune systems.
Food and aeroallergen sensitisation
Univariate analysis showed that food sensitisation,
but not aeroallergen sensitisation, was significantly
associated with a positive BCT result in patients with
AD (P=0.027). The association was not statistically
significant following regression analysis. It has been
reported that 40% of patients with food allergy, but
no diagnosis of asthma, have a substantial degree of
bronchial hyperactivity (measured by the BCT).31
Another small study (n=22 patients with allergic
asthma) showed no relationship between skin prick
test sensitisation and inhaled reactivity (measured
by the methacholine BCT).32 Skin allergen
sensitisation does not accurately predict airway
allergen response.32 33 This result was confirmed
by several other studies,34 35 which showed no
relationship between methacholine responsiveness
and the presence or degree of atopy. Hence, food
and aeroallergen sensitisation are generally not
associated with BCT results.
Serum immunoglobulin E level and blood
eosinophil count
Univariate analysis showed significant associations
of serum IgE level (P=0.045) and blood eosinophil
count (P=0.017) with a positive BCT result. These
findings were consistent with the results published
by Liu et al36 concerning significant relationships of BHR to methacholine and increased total serum
IgE to a positive BCT result (P=0.001). Sears et al37
showed that BHR was related to serum IgE level in
children not diagnosed with asthma; this relationship
persisted despite the exclusion of children with a
history of AR or AD. Hence, BHR is dependent on
serum IgE level and is unrelated to the presence of
AD. Nevertheless, IgE can be measured in young
children when BHR is difficult to demonstrate;
notably, IgE is a simple blood marker of AD severity
and asthma risk.7 38
Personal history of allergic rhinitis
The “one airway” hypothesis (ie, “united airway disease”) is based on the bidirectional interaction
between asthma and rhinitis, with the implication
that both upper and lower airways should be treated
for optimal symptomatic control.39 40 However, our
study showed no association between the presence
of AR and a positive BCT result in children with
AD (P=0.079). Although several studies have shown
an association between AR and BHR, they did not
equate a positive BCT result with the diagnosis of
AR.20 41 42
Atopic dermatitis severity
In our study, there was no association between AD
severity (based on objective SCORAD score, SH,
and TEWL) and a positive BCT result. Liu et al36 found that BHR to methacholine was related to
atopy (P=0.0063), while the degree of BHR was
not significantly associated with the severity of any
atopic disease.
Strengths and limitations of this study
The strengths of this study included collection and
review of the data over 10 years, which enabled the
addition of follow-up assessments for many of the
patients. The Prince of Wales Hospital is a public
hospital; the family cost of healthcare service is
relatively low, which supports a low dropout rate
and consistent long-term follow-up. Our relatively
large sample size enabled examination of various
atopic phenotypes. Patients with AD who exhibited
both skin and airway manifestations confirm the
presumed atopic march with progression from AD
to AR and asthma, or co-expression of asthma and
AD phenotypes (ie, skin sensitivity plus wheeze).
The primary limitation of the present study
was that data were missing for some factors, notably
family atopic conditions and laboratory tests.
Regarding the family history, many parents did not
provide clear information concerning their own
atopic conditions. Those with mild symptoms may
fail to seek medical advice, although they reported
self-diagnosed atopic conditions. Although only
physician-diagnosed conditions were included in the data analysis, there was difficulty confirming
the validity of those family histories. A substantial
number of patients were only children in their
families and had no siblings. Additionally, the BCT
is a medical test that provokes airway narrowing.
It is physically demanding and can cause severe
discomfort (eg, violent coughing) which makes the
measurement difficult. The irritating nature of the
BCT makes it suitable solely for older children and
causes high failure rates; thus, the number of patients
with a positive BCT result was relatively low. Our
observations concerning the usefulness of the BCT
should be confirmed in a prospective study.
Conclusions
In patients with paediatric AD, a positive BCT result
was independently and positively associated with
personal history of asthma and sibling history of
atopy, but not with any other clinical parameters.
Author contributions
Concept or design: KL Hon, AHY Ng, CCC Chan, PXY Ho, EPM Tsoi, TF Leung.
Acquisition of data: AHY Ng, CCC Chan, PXY Ho, EPM Tsoi.
Analysis or interpretation of data: KL Hon, AHY Ng, CCC Chan, PXY Ho, EPM Tsoi, KYC Tsang.
Drafting of the manuscript: KL Hon, AHY Ng, CCC Chan, PXY Ho, EPM Tsoi, FW Ko.
Critical revision of the manuscript for important intellectual content: KL Hon, FW Ko, TF Leung.
Acquisition of data: AHY Ng, CCC Chan, PXY Ho, EPM Tsoi.
Analysis or interpretation of data: KL Hon, AHY Ng, CCC Chan, PXY Ho, EPM Tsoi, KYC Tsang.
Drafting of the manuscript: KL Hon, AHY Ng, CCC Chan, PXY Ho, EPM Tsoi, FW Ko.
Critical revision of the manuscript for important intellectual content: KL Hon, FW Ko, TF Leung.
Conflicts of interest
As the editor of the journal, KL Hon was not involved in the peer review process. Other authors have disclosed no conflicts
of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by The Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research
Ethics Committee (The Joint CUHK-NTEC CREC).
References
1. Hon KL, Liu M, Zee B. Airway disease and environmental aeroallergens in eczematics approaching adulthood.
Pediatr Respirol Crit Care Med 2017;1:81-5. Crossref
2. Hon KL, Wang SS, Leung TF. The atopic march: from skin to the airways. Iran J Allergy Asthma Immunol 2012;11:73-7.
3. Hon KL, Yong V, Leung TF. Research statistics in atopic eczema: what disease is this? Ital J Pediatr 2012;38:26. Crossref
4. Coates AL, Wanger J, Cockcroft DW, et al. ERS technical standard on bronchial challenge testing: general
considerations and performance of methacholine challenge
tests. Eur Respir J 2017;49:1601526. Crossref
5. Dixon C. The bronchial challenge test: a new direction in asthmatic management. J Natl Med Assoc 1983;75:199-204.
6. Pepys J, Hutchcroft BJ. Bronchial provocation tests in etiologic diagnosis and analysis of asthma. Am Rev Respir
Dis 1975;112:829-59.
7. Ng C, Hon KL, Kung JS, Pong NH, Leung TF, Wong CK. Hyper IgE in childhood eczema and risk of asthma in
Chinese children. Molecules 2016;21:E753. Crossref
8. Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J
2005;26:153-61. Crossref
9. Ramsdale EH, Morris MM, Roberts RS, Hargreave FE. Bronchial responsiveness to methacholine in chronic
bronchitis: relationship to airflow obstruction and cold air
responsiveness. Thorax 1984;39:912-8.Crossref
10. Crapo RO, Casaburi R, Coates AL, et al. Guidelines for
methacholine and exercise challenge testing-1999. This
official statement of the American Thoracic Society was
adopted by the ATS Board of Directors, July 1999. Am J
Respir Crit Care Med 2000;161:309-29. Crossref
11. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38.Crossref
12. Spector SL, Farr RS. A comparison of methacholine and histamine inhalations in asthmatics. J Allergy Clin
Immunol 1975;56:308-16. Crossref
13. Schulze J, Smith HJ, Fuchs J, et al. Methacholine challenge in young children as evaluated by spirometry and impulse
oscillometry. Respir Med 2012;106:627-34. Crossref
14. Hon KL, Kung JS, Tsang KY, Yu JW, Cheng NS, Leung TF.
Do we need another symptom score for childhood eczema?
J Dermatolog Treat 2018;29:510-4. Crossref
15. Severity scoring of atopic dermatitis: the SCORAD index.
Consensus Report of the European Task Force on Atopic
Dermatitis [editorial]. Dermatology 1993;186:23-31. Crossref
16. Hon KL, Wong KY, Leung TF, Chow CM, Ng PC.
Comparison of skin hydration evaluation sites and
correlations among skin hydration, transepidermal water
loss, SCORAD index, Nottingham eczema severity score,
and quality of life in patients with atopic dermatitis. Am J
Clin Dermatol 2008;9:45-50. Crossref
17. Rothe T, Spagnolo P, Bridevaux PO, et al. Diagnosis and management of asthma—the Swiss guidelines. Respiration
2018;95:364-80. Crossref
18. Cockcroft DW. Direct challenge tests: Airway
hyperresponsiveness in asthma: its measurement and
clinical significance. Chest 2010;138(2 Suppl):18S-24S. Crossref
19. Huang SJ, Lin LL, Chen LC, et al. Prevalence of airway hyperresponsiveness and its seasonal variation in children
with asthma. Pediatr Neonatol 201;59:561-6. Crossref
20. Davis BE, Cockcroft DW. Past, present and future uses of methacholine testing. Expert Rev Respir Med 2012;6:321-9. Crossref
21. Pols DH, Wartna JB, van Alphen EI, et al. Interrelationships between atopic disorders in children: a meta-analysis based
on ISAAC questionnaires. PLoS One 2015;10:e0131869. Crossref
22. Riiser A, Hovland V, Carlsen KH, Mowinckel P, Lødrup Carlsen KC. Does bronchial hyperresponsiveness in
childhood predict active asthma in adolescence? Am J
Respir Crit Care Med 2012;186:493-500. Crossref
23. Toelle BG, Xuan W, Peat JK, Marks GB. Childhood factors that predict asthma in young adulthood. Eur Respir J
2004;23:66-70. Crossref
24. Küster W, Petersen M, Christophers E, Goos M, Sterry W. A family study of atopic dermatitis. Clinical and genetic
characteristics of 188 patients and 2151 family members.
Arch Dermatol Res 1990;282:98-102. Crossref
25. Johnson CC, Ownby DR, Peterson EL. Parental history of atopic disease and concentration of cord blood IgE. Clin
Exp Allergy 1996;26:624-9. Crossref
26. Thomsen SF, Ulrik CS, Kyvik KO, Ferreira MA, Backer V. Multivariate genetic analysis of atopy phenotypes in a
selected sample of twins. Clin Exp Allergy 2006;36:1382-90. Crossref
27. Ober C, Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun 2006;7:95-
100. Crossref
28. Sublett JL. The environment and risk factors for atopy. Curr Allergy Asthma Rep 2005;5:445-50.Crossref
29. Subbarao P, Mandhane PJ, Sears MR. Asthma: epidemiology, etiology and risk factors. CMAJ 2009;181:E181-90. Crossref
30. Goldberg S, Israeli E, Schwartz S, et al. Asthma prevalence, family size, and birth order. Chest 2007;131:1747-52 Crossref
31. Kivity S, Fireman E, Sade K. Bronchial hyperactivity,
sputum analysis and skin prick test to inhalant allergens in
patients with symptomatic food hypersensitivity. Isr Med
Assoc J 2005;7:781-4.
32. Bowton DL, Fasano MB, Bass DA. Skin sensitivity to
allergen does not accurately predict airway response to
allergen. Ann Allergy Asthma Immunol 1998;80:207-11. Crossref
33. Turner KJ, Stewart GA, Woolcock AJ, Green W, Alpers MP.
Relationship between mite densities and the prevalence
of asthma: comparative studies in two populations in the
Eastern Highlands of Papua New Guinea. Clin Allergy
1988;18:331-40. Crossref
34. Lúdvíksdóttir D, Janson C, Björnsson E, et al. Different airway responsiveness profiles in atopic asthma, nonatopic asthma, and Sjögren’s syndrome. BHR Study Group.
Bronchial hyperresponsiveness. Allergy 2000;55:259-65.Crossref
35. Suh DI, Lee JK, Kim CK, Koh YY. Methacholine and
adenosine 5’-monophosphate (AMP) responsiveness, and
the presence and degree of atopy in children with asthma.
Pediatr Allergy Immunol 2011;22:e101-6. Crossref
36. Liu SF, Lin MC, Chang HW. Relationship of allergic degree
and PC20 level in adults with positive methacholine
challenge test. Respiration 2005;72:612-6. Crossref
37. Sears MR, Burrows B, Flannery EM, Herbison GP,
Hewitt CJ, Holdaway MD. Relation between airway
responsiveness and serum IgE in children with asthma
and in apparently normal children. N Engl J Med
1991;325:1067-71. Crossref
38. Hon KL, Lam MC, Leung TF, et al. Are age-specific high
serum IgE levels associated with worse symptomatology
in children with atopic dermatitis? Int J Dermatol
2007;46:1258-62. Crossref
39. Haccuria A, Van Muylem A, Malinovschi A, Doan V,
Michils A. Small airways dysfunction: the link between
allergic rhinitis and allergic asthma. Eur Respir J
2018;51:1701749. Crossref
40. Lluncor M, Barranco P, Amaya ED, et al. Relationship
between upper airway diseases, exhaled nitric oxide, and
bronchial hyperresponsiveness to methacholine. J Asthma
2019;56:53-60. Crossref
41. Shaaban R, Zureik M, Soussan D, et al. Allergic rhinitis and
onset of bronchial hyperresponsiveness. Am J Respir Crit
Care Med 2007;176:659-66. Crossref
42. Ciprandi G, Signori A, Cirillo I. Relationship between
bronchial hyperreactivity and bronchodilation in patients
with allergic rhinitis. Ann Allergy Asthma Immunol
2011;106:460-6. Crossref