Hong Kong Med J 2021 Feb;27(1):18–26 | Epub 4 Feb 2021
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Paediatric glaucoma in Hong Kong: a multicentre
retrospective analysis of epidemiology, presentation, clinical interventions, and outcomes
Nafees B Baig, FHKAM (Ophthalmology), FCOphth HK1,2,3 #; Joyce J Chan, FHKAM (Ophthalmology), FCOphth HK1; Jonathan C Ho, FHKAM (Ophthalmology), FCOphth HK4; Geoffrey C Tang, MB, BS5; Susanna Tsang, FHKAM (Ophthalmology), FCOphth HK2; Kelvin H Wan, MB, ChB6 #; Wilson W Yip, FHKAM (Ophthalmology), FCOphth HK3,7; Clement CY Tham, FHKAM (Ophthalmology), FCOphth HK1,3
1 Hong Kong Eye Hospital, Kowloon Central Cluster, Hospital Authority, Hong Kong
2 Department of Ophthalmology, Kowloon West Cluster, Hospital Authority, Hong Kong
3 Department of Ophthalmology and Visual Sciences, The Chinese University of Hong Kong, Hong Kong
4 Department of Ophthalmology, Hong Kong East Cluster, Hospital Authority, Hong Kong
5 Department of Ophthalmology, Kowloon East Cluster, Hospital Authority, Hong Kong
6 Department of Ophthalmology, New Territories West Cluster, Hospital Authority, Hong Kong
7 Department of Ophthalmology and Visual Sciences, New Territories East Cluster, Hospital Authority, Hong Kong
# NB Baig is currently affiliated with: (1) Department of Ophthalmology,
Hong Kong Sanatorium and Hospital, Hong Kong and (2) Department
of Ophthalmology and Visual Sciences, The Chinese University of Hong
Kong, Hong Kong. JJ Chan is currently affiliated with Department of
Ophthalmology and Visual Sciences, The Chinese University of Hong
Kong, Hong Kong. JC Ho is currently affiliated with Clarity Medical Group
(Central), Hong Kong. KH Wan is currently affiliated with Department of Ophthalmology and Visual Sciences, The Chinese University of Hong
Kong, Hong Kong.
Corresponding author: Prof Clement CY Tham (clemtham@cuhk.edu.hk)
Abstract
Purpose: To document the epidemiology, presentation, clinical interventions, and outcomes of
paediatric glaucoma in Hong Kong.
Methods: This multicentre territory-wide
retrospective study was performed by reviewing
charts of patients with paediatric glaucoma in six
clusters of the Hong Kong Hospital Authority and The
Chinese University of Hong Kong from 2006 to 2015.
Results: This study included 150 eyes of
98 patients with paediatric glaucoma (presenting
age: 5.2±5.7 years). Of them, 35 eyes (23.3%) had
primary congenital glaucoma, 22 eyes (14.7%) had
juvenile open-angle glaucoma, and 93 eyes (62.0%)
had secondary glaucoma. The most prevalent types of
secondary glaucoma were lens-related after cataract
extraction (18.0%), Axenfeld–Rieger anomaly (5.3%),
uveitis (5.3%), Sturge–Weber syndrome (4.7%),
and traumatic (3.3%). The most common clinical
presentations were parental concerns (20.7%)
including cloudy cornea (12.7%) and tearing/photophobia (8.0%), followed by poor visual acuity
(18.0%), high intraocular pressure (13.3%), and
strabismus (6.0%). The follow-up duration was
8.46±6.51 years. Furthermore, 63.2% of eyes with
primary glaucoma and 45.2% of eyes with secondary
glaucoma were treated surgically. The final visual
acuity was 0.90±0.98 LogMAR; intraocular pressure
was 18.4±6.6 mm Hg; and number of glaucoma
medications was 2.22±1.61.
Conclusion: Primary congenital glaucoma was most
prevalent, followed by juvenile open-angle glaucoma
and aphakic glaucoma. Most eyes with primary
glaucoma required surgical treatment. Parental
concerns were important clinical presentations. Basic assessments by healthcare providers to
identify glaucoma signs (eg, poor visual acuity, high
intraocular pressure, and strabismus) warranted
prompt referral to an ophthalmologist.
New knowledge added by this study
- Primary congenital glaucoma and juvenile open-angle glaucoma are the most prevalent types of paediatric glaucoma in Hong Kong.
- While most patients with primary glaucoma required surgical intervention, most patients with secondary glaucoma were treated medically.
- Parental concerns were a critical factor in obtaining early medical attention. Basic ophthalmic assessments by healthcare providers warranted prompt referral to an ophthalmologist.
- Parental concerns regarding cloudy cornea, tearing, and photophobia are important clinical manifestations of paediatric glaucoma and are the chief complaints described to paediatricians, family physicians, or nurses.
- Prompt and basic ophthalmic assessments by healthcare providers, which identify signs of paediatric glaucoma (eg, poor visual acuity, ocular asymmetry, strabismus, nystagmus, and leukocoria), warrant early and rapid referral to an ophthalmologist.
Introduction
Paediatric glaucoma affects infants and children and
may result in irreversible blindness that substantially
diminishes productivity and quality of life over the
entire lifetime of affected individuals. Prognosis is
largely dependent on early, accurate diagnosis and
timely treatment, comprising rigorous intraocular
pressure (IOP) reduction to a level at which further
progression is unlikely; the prevention of amblyopia
is also a critical component of treatment.1 Paediatric
glaucoma is classified as ‘primary’ when it involves
an isolated idiopathic developmental abnormality of
the anterior chamber angle, whereas it is classified
as ‘secondary’ when aqueous outflow is reduced
because of a congenital or acquired ocular disease or
systemic disorder.2
Primary paediatric glaucoma includes
primary congenital glaucoma (PCG, isolated
trabeculodysgenesis) and juvenile open-angle
glaucoma. Primary congenital glaucoma is the
most common type of glaucoma in infants,3 4 with
a variable incidence reported worldwide. Higher
incidences have been observed in inbred populations
where parental consanguinity is common.5 6 7 Primary congenital glaucoma occurs more frequently in
boys than in girls8 9 10; it is bilateral in 70% to 80% of
patients.11 12 Patients with familial PCG tend to have
an equal sex distribution.10 11 13
Secondary paediatric glaucoma is commonly
associated with anterior segment dysgenesis; 50% of
patients develop glaucoma.14 Glaucoma associated
with aniridia is usually caused by progressive angle
closure; it presents often in childhood with an
incidence of 6% to 75% in aniridic eyes.15 Aphakic
glaucoma can occur soon or years after initial
uneventful cataract extraction surgery in children
with congenital cataract; its incidence ranges
from 5% to 41%, depending on patient age at the
time of surgery, corneal diameter, and surgical
techniques.16 17 18 19 Phacomatoses commonly associated
with glaucoma include Sturge–Weber syndrome20
and Klippel–Trenaunay–Weber syndrome. The
glaucoma evident in patients with inflammatory
disorders is multifactorial, with a reported incidence
of up to 38% in children with juvenile idiopathic
arthritis.21
The primary goal of treatment for both primary
and secondary types of paediatric glaucoma is IOP
reduction, for which medical treatment is often the
first-line approach. Longer-term treatment involves
surgery as the definitive approach for IOP control
in the vast majority of patients with paediatric
glaucoma. Available surgical procedures have various
indications, with both advantages and disadvantages,
as well as different success rates, among patient
populations. Notably, the management approach and
success rate also considerably vary among countries
worldwide. Paediatric congenital glaucoma is a relatively uncommon disease, such that a consultant
ophthalmologist in a general ophthalmology centre
in the Western world is estimated to encounter a new
patient with PCG approximately once every 5 years.22
Because of its relative rarity, PCG is sometimes
misdiagnosed or not treated appropriately,
especially in general ophthalmology centres, leading
to irreversible corneal and optic nerve damage,
as well as unnecessary irreversible visual loss.
Consequently, PCG is present in a disproportionate
percentage (up to 18%) of children in institutions
for the blind worldwide.23 24 Furthermore, congenital
glaucoma was reportedly present in 30% of paediatric
patients attending a university low vision service.25
Overall, paediatric glaucoma is responsible for 5%
of irreversible blindness in children worldwide.26
However, there is a paucity of contemporary
epidemiologic and clinical data regarding paediatric
glaucoma in Hong Kong.
We conducted the Hong Kong Paediatric
Glaucoma Study as the first territory-wide
analysis of the epidemiology, presentation, clinical
interventions, and outcomes of paediatric glaucoma
in Hong Kong. This study is expected to greatly
enhance the understanding of this disease in our
local community, while improving our disease
management approaches and standards of clinical
care. The findings will also provide our colleagues
in Paediatrics and Family Medicine with a clearer
overview of the clinical presentations of patients
with paediatric glaucoma.
Methods
Study design and ethical approval
This study comprised a retrospective chart review
of patients with confirmed paediatric glaucoma
who were managed over a 10-year period (January
2006 to December 2015) in the ophthalmology
departments of six regional clusters of the Hospital
Authority in Hong Kong (ie, Hong Kong East,
Kowloon West, Kowloon Central, Kowloon East,
New Territories West, and New Territories East)
and The Chinese University of Hong Kong. The
Hospital Authority in Hong Kong provides a heavily
government-subsidised public clinical service to all
Hong Kong citizens, while the Hospital Authority
ophthalmology service provides more than 90% of
all clinical ophthalmology services delivered in Hong
Kong. The six hospital clusters participating in this
study had a total population of 6 889 400 in 2017,
which represented 92.96% of the total population
(7 411 300) in Hong Kong at the time of the study.27
This study was performed in accordance with the
1996 Declaration of Helsinki and ICH-GC; the study
protocol was approved by the institutional review
boards of all involved clusters.
Patient population
Using the Hospital Authority’s Clinical Data
Analysis and Reporting System, we identified
patients aged ≤18 years on presentation, all of whom
had either undergone glaucoma surgery or been
prescribed glaucoma medication(s) continuously for
>3 months. Patients identified through the Clinical
Data Analysis and Reporting System were then
verified through the Clinical Management System,
which is an electronic medical records system in use
throughout all hospitals and departments under the
Hospital Authority in Hong Kong. Hard copies of
medical records were also collected and reviewed to
ensure the patients met the following criteria:
1. Age ≤18 years at presentation, with a diagnosis of
primary or secondary glaucoma;
2. A combination of previous and/or current high IOP (>21 mm Hg), combined with disc cupping >0.3 or disc asymmetry >0.2, as well as one or more of the following signs: progressive disc cupping, buphthalmos (prominent, enlarged eye), enlarged corneal diameter (>11 mm in newborns, >12 mm in children aged <1 year, or >13 mm in children of any age), corneal oedema, Descemet’s membrane splitting (Haab’s striae), visual field defects, or progressive myopia.
2. A combination of previous and/or current high IOP (>21 mm Hg), combined with disc cupping >0.3 or disc asymmetry >0.2, as well as one or more of the following signs: progressive disc cupping, buphthalmos (prominent, enlarged eye), enlarged corneal diameter (>11 mm in newborns, >12 mm in children aged <1 year, or >13 mm in children of any age), corneal oedema, Descemet’s membrane splitting (Haab’s striae), visual field defects, or progressive myopia.
Data collection
Clinical data of all patients who met the above study
criteria were retrospectively collected from medical
records and the Clinical Management System, using
standardised data sheets. The following data were collected: patient demographics including family
history of glaucoma and parental consanguinity
(defined as a union between two related individuals
who were second cousins or closer), type of glaucoma
(primary/secondary), presentation of disease/reason
for referral, examination findings on presentation,
subsequent management (eg, medications, laser
interventions, and surgical interventions), and
clinical outcomes at the final follow-up. Patients’
Hong Kong Identity Card numbers were used to
identify duplicate entries at different hospitals; in
such instances, the clinical data were combined
prior to analysis.
Outcome measures
The primary outcome measures were the
epidemiological characteristics and clinical
presentations of patients with paediatric glaucoma
in Hong Kong. The secondary outcome measures
were the subsequent management of these patients
and their clinical outcomes at the final follow-up.
Results
Patient characteristics and epidemiological
findings
In this study, we identified 98 patients with paediatric
glaucoma (150 eyes; 47 boys and 51 girls). Seventy
eyes (46.7%) were right eyes, and the mean ± standard
deviation (SD) presenting age was 5.2±5.7 years
(range, 0-18 years). With the exception of two
patients (one Japanese and one from mid-western
Asia), all included patients were of Chinese ethnic
origin. Three patients (3.1%) had a positive family
history of glaucoma, while none had parental
consanguinity. The mean ± SD duration of follow-up
was 8.46±6.51 years (range, 0.2-25.5 years). While one
patient had pigment dispersion syndrome (follow-up
duration of 2 months) and one patient had persistent
hyperplastic primary vitreous (follow-up duration
of 5 months), all other included patients had a
minimum follow-up duration of 6 months. The Hong
Kong population aged <20 years was 1 378 912 in
2006,28 and it was 1 174 500 in 2015.29 The population
covered by the involved six Hospital Authority
clusters and The Chinese University of Hong Kong
eye clinic constituted approximately 93% of the
total population.27 Given that Hospital Authority
ophthalmology departments provided services
to 90% of our general population, the estimated
annual incidence rate of paediatric glaucoma in our
Hong Kong was 0.92 per 100 000 population aged
<20 years.
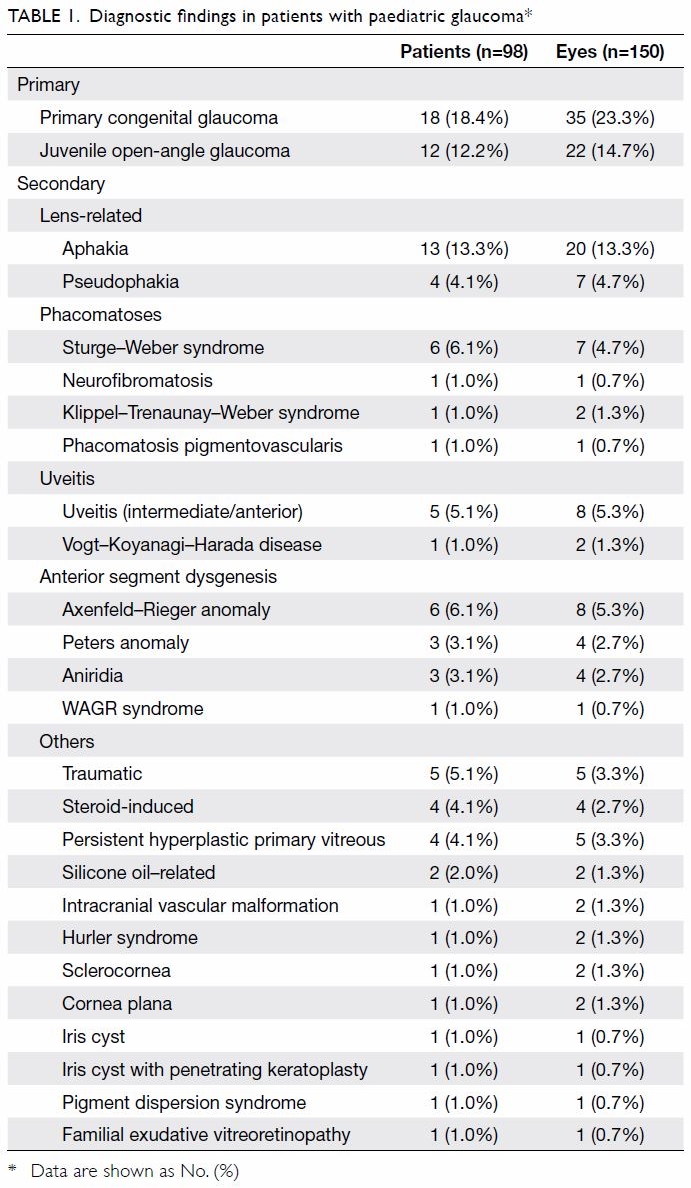
Types of glaucoma and presenting symptoms
Among the patients in this study, 57 eyes of
30 patients had primary glaucoma (35 eyes of
18 patients had PCG and 22 eyes of 12 patients had juvenile open-angle glaucoma). Furthermore, 93 eyes
of 68 patients had secondary glaucoma (Table 1).
The most prevalent type of secondary glaucoma was
lens-related glaucoma after cataract extraction for
congenital cataract (27 eyes of 17 patients, 18.0% of
all involved eyes), which included aphakic glaucoma
(13.3%) and pseudophakic glaucoma (4.7%). Other
types of secondary glaucoma were Axenfeld–Rieger
anomaly (8 eyes, 5.3%), uveitis (intermediate/anterior, 8 eyes, 5.3%), Sturge–Weber syndrome (7 eyes, 4.7%), and traumatic
(5 eyes, 3.3%).
The main presenting symptoms are summarised
in Table 2. Common clinical presentations were
parental concerns (31 eyes of 21 patients, 20.7% of
all involved eyes) including cloudy cornea (19 eyes
of 13 patients, 12.7%) and tearing/photophobia
(12 eyes of 8 patients, 8.0%); other presentations
that warranted referral to an ophthalmologist
included poor visual acuity (27 eyes, 18.0%), high
IOP (20 eyes, 13.3%), and strabismus (9 eyes, 6.0%).
The mean ± SD IOP on presentation to the attending
ophthalmologist was 25.3±10.2 mm Hg (range,
7-53 mm Hg). Notably, one eye had an iris cyst and
underwent penetrating keratoplasty; although its
IOP was 7 mm Hg, it showed an increased cup-to-disc
ratio (0.5) and was therefore included in the cohort.
The mean ± SD visual acuity was 0.6±0.7 logarithm
of the minimum angle of resolution (LogMAR;
range, -0.08 to 3.00), the mean ± SD spherical
equivalent was -2.5±5.6 (range, -12.6 to 13.3),
and the mean ± SD cup-to-disc ratio was 0.59±0.22
(range, 0.2-1.0).
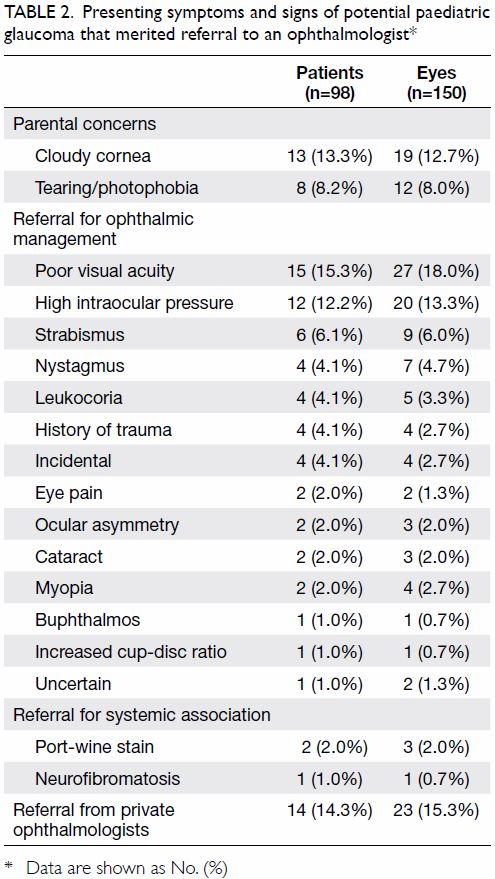
Table 2. Presenting symptoms and signs of potential paediatric glaucoma that merited referral to an ophthalmologist
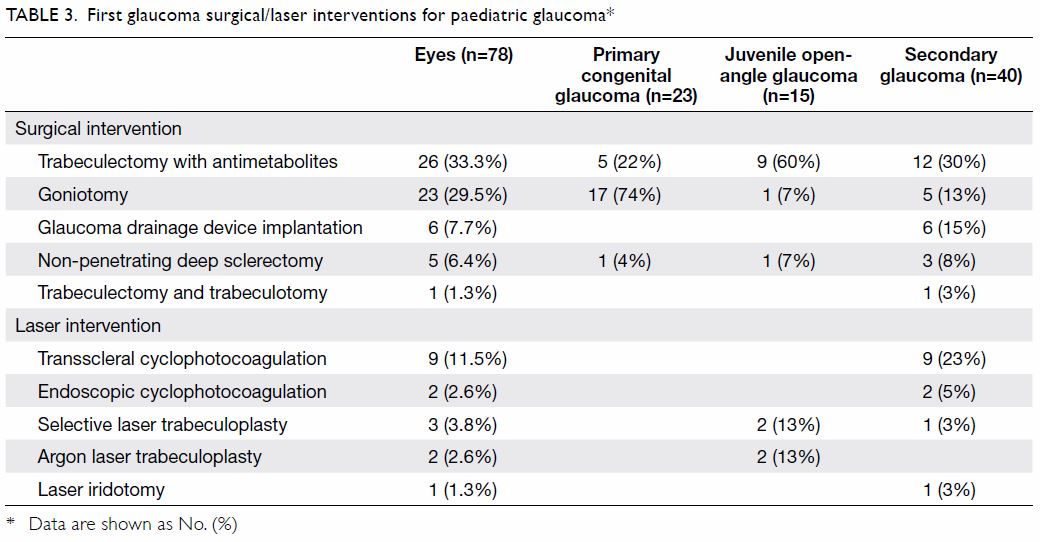
Glaucoma interventions
Among the 78 eyes which underwent surgical
or laser interventions, 38 (48.7%) had primary
glaucoma and 40 (51.3%) had secondary glaucoma.
The first glaucoma intervention for each eye is
indicated in Table 3. The most commonly performed
surgery was trabeculectomy with antimetabolites
(26 eyes, 33.3%) followed by goniotomy (23 eyes,
29.5%) and glaucoma drainage device implantation
(6 eyes, 7.7%). The most commonly performed laser
procedure was transscleral cyclophotocoagulation
(9 eyes, 11.5%). The mean ± SD number of glaucoma
interventions per eye was 1.37±1.90 (range, 0-9).
Of the 78 eyes which underwent surgical or laser
interventions, 31 (39.7%) received one intervention
during the study period while 47 (60.3%) received
more than one intervention.
In all, 63.2% of eyes with primary glaucoma
were treated surgically during the follow-up period;
54.8% of eyes with secondary glaucoma were treated
by medications alone during the follow-up period.
Among the 35 eyes of 18 patients with PCG, 23 eyes
(66%) were managed surgically and only six of
them (17.1%) were medication-free on the final
follow-up. Among the 22 eyes of 12 patients with juvenile open-angle glaucoma, 15 eyes (68%) were
managed surgically and only six of them (27.3%)
were medication-free on the final follow-up. Among
the 93 eyes of 68 patients with secondary glaucoma,
40 eyes (43.0%) were managed surgically during
follow-up and 14 (15.1%) were medication-free on
the final follow-up.
Follow-up findings
The mean ± SD LogMAR visual acuity at the final
follow-up was 0.90±0.98 (range, -0.19 to 3.00, ie,
no light perception). Among 111 eyes for which visual acuity was determined, 10 (9.0%) had no light
perception at the final follow-up, four (3.6%) had
light perception, five (4.5%) could perceive hand
movement, and four (3.6%) could perceive finger
counting. The mean ± SD IOP at the final follow-up
was 18.4±6.6 mm Hg (range, 6-43 mm Hg), while
the mean ± SD number of glaucoma medications
at the final follow-up was 2.22±1.61 (range, 0-5).
The mean ± SD spherical equivalent was -3.4±6.6
(range, -18.25 to 13.1), whereas the mean ± SD
cup-to-disc ratio was 0.68±0.20 (range, 0.3-1.0).
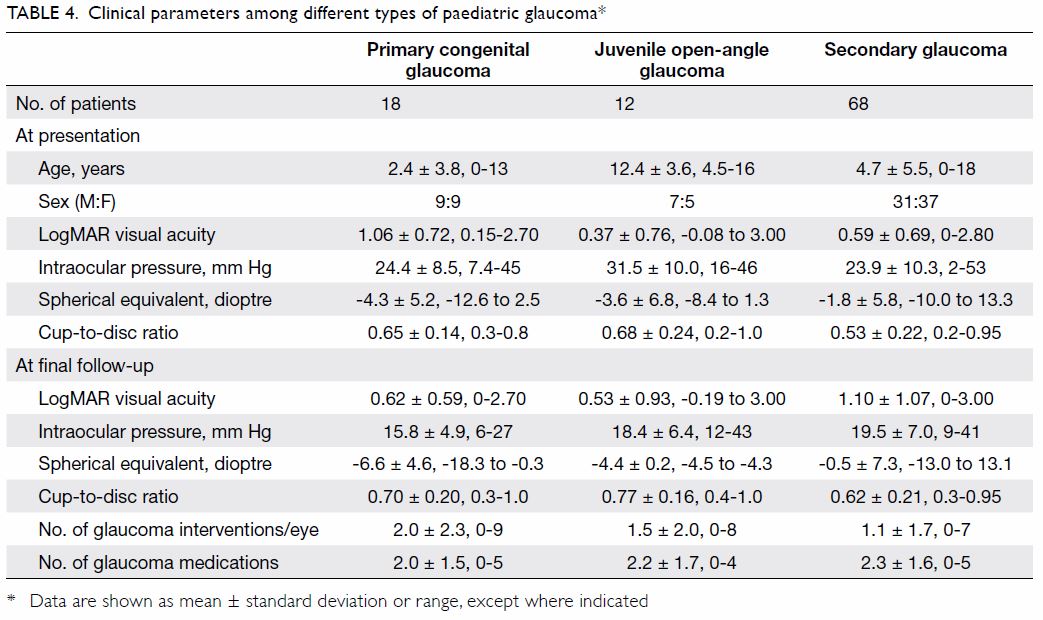
Clinical parameters among the different types of
glaucoma (ie, PCG, juvenile open-angle glaucoma,
and secondary glaucoma) are described in Table 4.
As expected, PCG manifested at an earlier age,
compared with other types of glaucoma. Patients
with secondary glaucoma had a wider range of
refraction because some exhibited hyperopia, such
as in aphakic glaucoma. Other parameters (eg, IOP,
cup-to-disc ratio, number of glaucoma interventions,
and number of medications) were similar among the
different types of glaucoma.
Discussion
Effects of ethnicity on paediatric glaucoma
incidence and type
To our knowledge, this is the first epidemiological
report concerning paediatric glaucoma in Hong
Kong. Hong Kong had a population of 7.4 million in
2018,30 of which 92.0% were of Chinese ethnic origin,
while there were 2.5% Filipinos, 2.1% Indonesians,
and 0.8% Caucasians.29 In this study, we included patients with confirmed paediatric glaucoma who
were managed in the ophthalmology departments of
six regional clusters of the Hospital Authority in Hong
Kong (Hong Kong East, Kowloon West, Kowloon
Central, Kowloon East, New Territories West, and
New Territories East) and The Chinese University of
Hong Kong. In this study, we estimated the annual
incidence rate of paediatric glaucoma in Hong Kong
to be 0.92 per 100 000 population <20 years of age.
The reported incidence rates have varied among
previous studies, presumably because of differences
in the ethnicity of the study population. In the
British Infantile and Childhood Glaucoma Eye Study,
Papadopoulos et al31 concluded that the incidence of
PCG was nearly ninefold greater among children of
Pakistani origin, compared with Caucasian children;
other groups with high incidences of PCG included
those of Bangladeshi and Indian origin. Notably, only
one Chinese child (among 99 paediatric patients
with newly diagnosed glaucoma) was diagnosed with
PCG during the 1-year surveillance period. In total,
67% of all Pakistani children in that study were from
consanguineous marriages. Although South Asians
remain a minority in Hong Kong, local census data29
showed that the population increased from 47 505
to 80 028 from 2006 to 2016 (68% increase). Thus,
clinicians should be aware of the potential for this
condition among babies and children of specific
ethnic origins.
Our study showed that PCG was the most
prevalent type of glaucoma in our patient population,
present in 23.3% of the included eyes; other common
types were juvenile open-angle glaucoma (14.7%) and aphakic glaucoma (13.3%). The reported prevalences
have varied among types of paediatric glaucoma in
previous studies. Taylor et al3 described a population
of Canadian patients with paediatric glaucoma, in
which congenital glaucoma was the most common
subtype (38% of patients). In the British Infantile and
Childhood Glaucoma Eye Study,31 45 of 95 patients
(47.4%) were diagnosed with PCG during the
1-year surveillance period. In mainland China, two
hospital-based studies revealed the epidemiology of
paediatric glaucoma in Chinese populations.32 33 Both
studies concluded that congenital glaucoma was the
most common subtype. Aponte et al34 reported the
40-year incidence and clinical characteristics of
childhood glaucoma among patients in the region
of Rochester, United States. They concluded that
acquired and secondary forms of glaucoma were
the most common, while congenital and juvenile
forms of glaucoma were rare. Thus, we presume that
variations in prevalence among different types of
glaucoma are related to ethnicity.
Potential mechanisms underlying paediatric
glaucoma
Among patients with secondary glaucoma, the most
commonly associated conditions were lens-related:
aphakia (13.3% of eyes) and pseudophakia (4.7%)
after cataract extraction for congenital cataract.
Although the exact mechanisms of glaucoma in
young patients with aphakia and pseudophakia
are not well known, Beck et al35 suggested the
following aetiologies based on their findings in the Infant Aphakia Treatment Study: congenital angle
anomalies, postoperative inflammation leading to
angle dysfunction or progressive synechial closure,
corticosteroid-induced mechanisms, and some
unknown influences of the aphakic state or vitreous
interaction with developing angle structures that
cause reduced outflow.
Congenital conditions, such as anterior
segment dysgenesis (11.4%) and Sturge–Weber
syndrome (4.7%), were also associated with glaucoma
in our patients. Clinicians could occasionally discern
irregular pupils or abnormal red reflex from the
fundi in patients with anterior segment dysgenesis;
Sturge–Weber syndrome is associated with the
presence of a facial port-wine stain.
The mechanisms of uveitic glaucoma are
not clearly known, they may involve inflammatory
substances/cellular components that cause
trabecular damage and blockage, as well as a
response to steroid treatment in young patients,
which causes high IOP.36 In addition to uveitis,
2.7% of eyes had steroid-induced glaucoma related to
the chronic use of topical steroid treatment for other
ophthalmic conditions (eg, allergy and chalazion)
or systemic conditions (eg, eczema). Therefore,
medication history concerning steroid use is an
important consideration in paediatric patients;
steroid self-medication and/or long-term steroid use
without close IOP monitoring could carry a risk of
glaucoma.37
Trauma-related glaucoma was also observed
in 3.3% of included eyes; four of the five patients
with traumatic glaucoma exhibited angle recession.
Therefore, IOP should generally be measured in
young patients after ocular trauma and the angle
structure should be examined via gonioscopy
whenever possible.
Ophthalmic complaints and early clinical
assessments of paediatric glaucoma
Parental concerns of tearing, photophobia, and
cloudy cornea comprised approximately 21.5%
of the reasons for referral in this cohort (20.7% of
total eyes). High IOP comprised only 12.2% of the
reasons for referral (13.3% of eyes); other ophthalmic
complaints leading to referral included poor visual
acuity (18.0% of eyes), strabismus (6.0% of eyes), and
nystagmus (4.7% of eyes); these complaints could
be related to the presence of unilateral or bilateral
amblyopia. Buphthalmia is a finding of glaucoma
in infancy as the young eye increases in size from
elevated IOP due to corneal and scleral collagen
immaturity (Fig).2 Therefore, a subset of patients
presented with ocular asymmetry, buphthalmos,
increased or early myopia, or increased cup-to-disc
ratio with or without elevated IOP. Furthermore,
although visual acuity may not be fully assessed
in babies and young children, there is a need to actively screen for and treat amblyopia in this patient
group. Amblyopia remains the main cause of poor
visual acuity in patients with paediatric glaucoma.
Appropriate correction of refractive error and
eye patching are essential components of clinical
management for these patients.
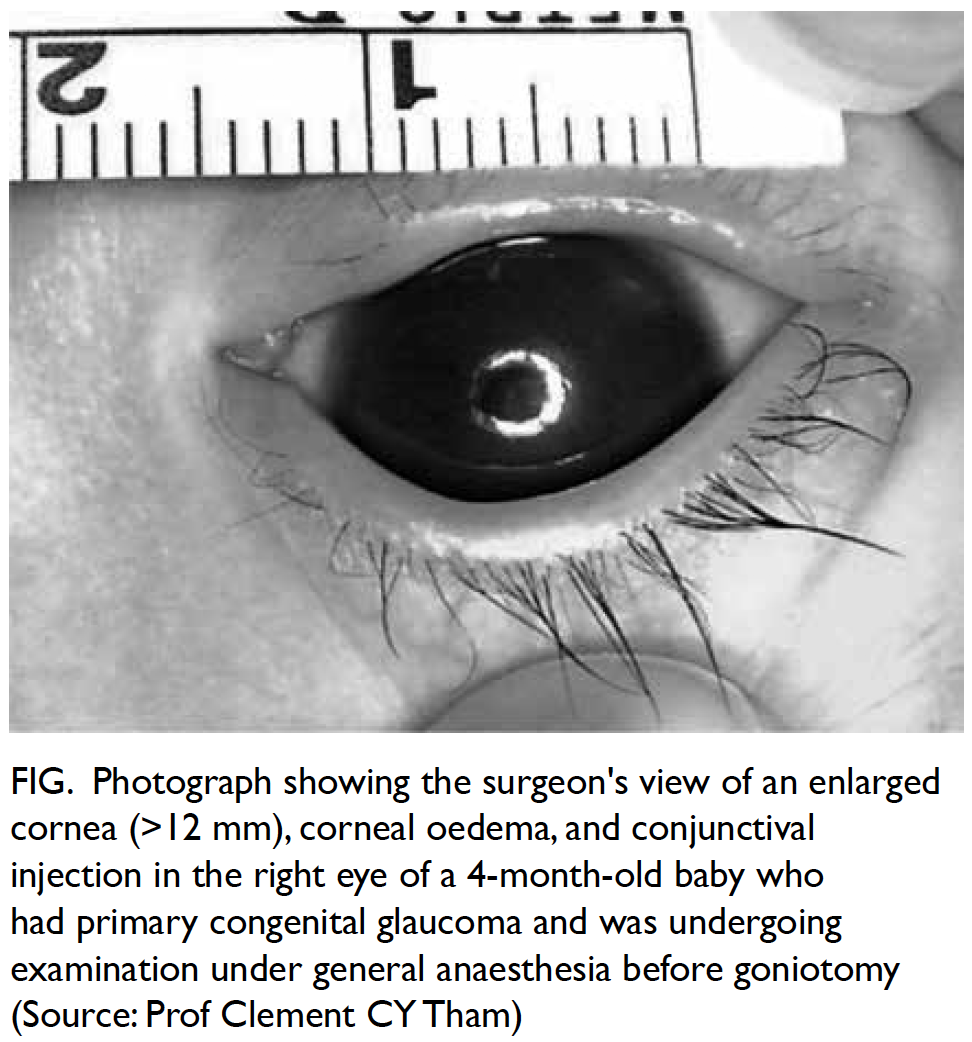
Figure. Photograph showing the surgeon’s view of an enlarged cornea (>12 mm), corneal oedema, and conjunctival injection in the right eye of a 4-month-old baby who had primary congenital glaucoma and was undergoing examination under general anaesthesia before goniotomy (Source: Prof Clement CY Tham)
Family doctors and paediatricians are usually
the first clinicians to examine paediatric patients
with suspected glaucoma in the healthcare setting
in Hong Kong. Early detection and diagnosis
are important for preventing the loss of vision.
Appropriate referral is based on the detection of
signs and symptoms of paediatric glaucoma. Clinical
signs include epiphora, conjunctival erythema,
corneal enlargement, corneal clouding, Haabs striae,
abnormally deep anterior chamber, myopia and/or
astigmatism, and enlarged optic nerve cupping.
The classic triad of epiphora, photophobia, and
blepharospasm is usually evident in patients with
congenital glaucoma. Other symptoms of paediatric
glaucoma often include a cloudy and enlarged cornea
or large eye, ocular asymmetry (ie, one eye larger than
the other), blurring, frequent eye rubbing, pain and
discomfort; moreover, the child may become irritable
and fussy, and may exhibit a poor appetite. Family
doctors and paediatricians may assess vision in older
babies or young children; measure IOP using non-contact
tonometry in older children (although there
is no established range of normal IOP in paediatric
patients, an IOP ≥21 mm Hg would merit referral to
an ophthalmologist); and may detect manifestations
of strabismus, nystagmus, ocular asymmetry,
cloudy cornea, irregular pupil, and/or conjunctival
injection. Prompt and early referrals are important
for minimising visual loss and preventing amblyopia.
Limitations
This study had some notable limitations. First,
paediatric glaucoma is a rare and diverse condition
with heterogeneous manifestations. Thus, there are
no widely established diagnostic criteria and no
standardised management protocol in use among
different hospitals. Second, clinical data were collected
retrospectively from the patients’ medical records in
this study. Because the ophthalmology departments
in most Hospital Authority service clusters have
not fully implemented the use of electronic medical
records for both out-patients and in-patients, the
retrospective collection of handwritten clinical
data from hard-copy medical records might have
led to the unintentional exclusion of patients or
data. Furthermore, missing data and recall bias
may have influenced the findings, especially with
respect to symptom presentation at the first clinical
visit. Among patients who had not received surgical
interventions, diagnostic information might have
been omitted for some patients in some clusters.
Finally, patients with paediatric glaucoma managed
in the Hong Kong West Cluster and in the private
sector were not included in this study; thus, the
findings may not have been entirely representative of
the whole territory, although this remains the largest
cohort study of patients with paediatric glaucoma in
Hong Kong.
Conclusion
Paediatric glaucoma remains an important and
irreversible blinding eye disease among children.
Children are often unable to complain of specific
symptoms and the signs of paediatric glaucoma
are often subtle; thus, parents, family doctors, and
paediatricians should be familiar with the common
manifestations of this disease. Family doctors
and paediatricians should have a very high level
of suspicion for paediatric glaucoma, combined
with a lower threshold for referring patients to
ophthalmologists for further evaluation and early
treatment. Among the known types of paediatric
glaucoma, PCG is the most common. Children with
unexplained cloudy corneas, tearing, photophobia,
diminished visual acuity, and signs of squinting
should be promptly referred for further assessment.
Author contributions
Concept or design: NB Baig and CC Tham.
Acquisition of data: JJ Chan, JC Ho, GC Tang, S Tsang, KH Wan, WK Yip.
Analysis or interpretation of data: NB Baig and CC Tham.
Drafting of the manuscript: NB Baig and CC Tham.
Critical revision of the manuscript for important intellectual content: NB Baig and CC Tham.
Acquisition of data: JJ Chan, JC Ho, GC Tang, S Tsang, KH Wan, WK Yip.
Analysis or interpretation of data: NB Baig and CC Tham.
Drafting of the manuscript: NB Baig and CC Tham.
Critical revision of the manuscript for important intellectual content: NB Baig and CC Tham.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The study obtained ethics approval from the following ethics committees:
Research Ethics Committee (Kowloon Central/Kowloon
East) (Ref: KC/KE-15-0013/ER-2);
Research Ethics Committee, Kowloon West Cluster (Ref:
KW/EX-15-056[85-09]);
Joint Chinese University of Hong Kong–New Territories
East Cluster Clinical Research Ethics Committee (Ref:
2015.100);
New Territories West Cluster Clinical & Research Ethics
Committee (Ref: NTWC/CREC/15010);
Hong Kong East Cluster Research Ethics Committee (Ref:
HKEC-2015-033).
The participants (or a legal guardian) gave informed consent before the study.
The participants (or a legal guardian) gave informed consent before the study.
References
1. Richardson KT Jr, Ferguson WJ Jr, Shaffer RN. Long-term functional results in infantile glaucoma. Trans Am Acad
Ophthalmol Otolaryngol 1967;71:833-7.
2. Papadopoulos M, Khaw PT. Childhood glaucoma. In:
Taylor D, Hoyt CS, editors. Pediatric Ophthalmology and
Strabismus. 3rd ed. Philadelphia: Elsevier Saunders; 2005:
458-71.
3. Taylor RH, Ainsworth JR, Evans AR, Levin AV. The
epidemiology of pediatric glaucoma: the Toronto
experience. J AAPOS 1999;3:308-15. Crossref
4. Shaffer RN, Weiss DI. Infantile glaucoma: diagnosis and
differential diagnosis. Congenital and Pediatric Glaucomas.
St. Louis: CV Mosby; 1970: 37-59.
5. Elder MJ. Congenital glaucoma in the West Bank and Gaza Strip. Br J Ophthalmol 1993;77:413-6. Crossref
6. Genĉík A. Epidemiology and genetics of primary congenital
glaucoma in Slovakia. Description of a form of primary
congenital glaucoma in gypsies with autosomal-recessive
inheritance and complete penetrance. Dev Ophthalmol
1989;16:76-115.
7. Turaçli ME, Aktan SG, Sayli BS, Akarsu N. Therapeutic and
genetical aspects of congenital glaucomas. Int Ophthalmol
1992;16:359-62. Crossref
8. McGinnity FG, Page AB, Bryars JH. Primary congenital glaucoma: twenty years experience. Ir J Med Sci
1987;156:364-5. Crossref
9. Jay MR, Rice NS. Genetic implications of congenital glaucoma. Metab Ophthalmol 1978;2:257-8.
10. Barsoum-Homsy M, Chevrette L. Incidence and prognosis of childhood glaucoma. A study of 63 cases. Ophthalmology
1986;93:1323-7. Crossref
11. François J. Congenital glaucoma and its inheritance. Ophthalmologica 1980;181:61-73. Crossref
12. Morin JD, Merin S, Sheppard RW. Primary congenital glaucoma—a survey. Can J Ophthalmol 1974;9:17-28.
13. Sarfarazi M, Stoilov I. Molecular genetics of primary congenital glaucoma. Eye (Lond) 2000;14:422-8. Crossref
14. Idrees F, Vaideanu D, Fraser SG, Sowden JC, Khaw PT. A review of anterior segment dysgeneses. Surv Ophthalmol
2006;51:213-31. Crossref
15. Nelson LB, Spaeth GL, Nowinski TS, Margo CE, Jackson L.
Aniridia. A review. Surv Ophthalmol 1984;28:621-42.Crossref
16. Francois J. Late results of congenital cataract surgery.
Ophthalmology 1979;86:1586-98. Crossref
17. Simon JW, Mehta N, Simmons ST, Catalano RA, Lininger LL. Glaucoma after pediatric lensectomy/vitrectomy.
Ophthalmology 1991;98:670-4. Crossref
18. Rabiah PK. Frequency and predictors of glaucoma after pediatric cataract surgery. Am J Ophthalmol 2004;137:30-7. Crossref
19. Vishwanath M, Cheong-Leen R, Taylor D, Russell-Eggitt I, Rahi J. Is early surgery for congenital cataract a risk factor
for glaucoma? Br J Ophthalmol 2004;88:905-10. Crossref
20. Sullivan TJ, Clarke MP, Morin JD. The ocular manifestations of the Sturge-Weber syndrome. J Pediatr Ophthalmol
Strabismus 1992;29:349-56.
21. Sijssens KM, Rothova A, Berendschot TT, de Boer JH. Ocular hypertension and secondary glaucoma in children
with uveitis. Ophthalmology 2006;113:853-9.e2. Crossref
22. Walton DS. Primary congenital open-angle glaucoma.
In: Chandler PA, Grant WM, editors. Glaucoma. 2nd ed.
Philadelphia: Lea & Febiger; 1979: 329-43.
23. Tabbara KF, Badr IA. Changing pattern of childhood blindness in Saudi Arabia. Br J Ophthalmol 1985;69:312-5. Crossref
24. Gilbert CE, Canovas R, Kocksch de Canovas R, Foster A.
Causes of blindness and severe visual impairment in
children in Chile. Dev Med Child Neurol 1994;36:326-33. Crossref
25. Haddad MA, Lobato FJ, Sampaio MW, Kara-José N.
Pediatric and adolescent population with visual
impairment: study of 385 cases. Clinics (Sao Paulo)
2006;61:239-46. Crossref
26. Gilbert CE, Rahi JS, Quinn GE. Visual impairment and
blindness in children. In: Johnson GJ, Minassian DC,
Weale RA, West SK, editors. The Epidemiology of Eye
Disease. 2nd ed. London: Edward Arnold Ltd; 2003: 260-86.
27. Planning Department, Hong Kong SAR Government. Projections of Population Distribution 2015-2024.
Hong Kong: Planning Department, Hong Kong SAR
Government; 2015.
28. Census and Statistics Department, Hong Kong SAR Government. Hong Kong 2006 Population By-Census
(Report). Hong Kong: Census and Statistics Department,
Hong Kong SAR Government; 2006.
29. Census and Statistics Department, Hong Kong SAR Government. Hong Kong 2016 Population By-Census
(Report). Hong Kong: Census and Statistics Department,
Hong Kong SAR Government; 2016.
30. Census and Statistics Department, Hong Kong SAR Government. Hong Kong Monthly Digest of Statistics
(Report). Hong Kong: Census and Statistics Department,
Hong Kong SAR Government; 2018.
31. Papadopoulos M, Cable N, Rahi J, Khaw PT, BIG Eye Study Investigators. The British Infantile and Childhood
Glaucoma (BIG) Eye Study. Invest Ophthalmol Vis Sci
2007;48:4100-6. Crossref
32. Qiao CY, Wang LH, Tang X, Wang T, Yang DY, Wang NL. Epidemiology of hospitalized pediatric glaucoma
patients in Beijing Tongren Hospital. Chin Med J (Engl)
2009;122:1162-6.
33. Fang Y, Long Q, Guo W, Sun X. Profile of pediatric glaucoma patients in Shanghai Eye, Ear, Nose and Throat
Hospital. Chin Med J (Engl) 2014;127:1429-33.
34. Aponte EP, Diehl N, Mohney BG. Incidence and clinical characteristics of childhood glaucoma: a population-based
study. Arch Ophthalmol 2010;128:478-82. Crossref
35. Beck AD, Freedman SF, Lynn MJ, Bothun E, Neely DE, Lambet SR, Infant Aphakia Treatment Study Group.
Glaucoma-related adverse events in the Infant Aphakia
Treatment Study: 1-year results. Arch Ophthalmol
2012;130:300-5. Crossref
36. Sen ES, Dick AD, Ramanan AV. Uveitis associated with juvenile idiopathic arthritis. Nat Rev Rheumatol
2015;11:338-48. Crossref
37. Nuyen B, Weinreb RN, Robbins SL. Steroid-induced glaucoma in the pediatric population. J AAPOS 2017;21:1-6. Crossref